
- Manuals
- Brands
- Braun Manuals
- Health Care Products
- Perfusor Space
- Instructions for use manual
-
Contents
-
Table of Contents
-
Bookmarks
Quick Links
Perfusor® Space
and Accessories
Instructions for Use
It is recommended that all pumps at
your care unit are equipped with the
same software version.
GB
Valid for software 688M
Related Manuals for Braun Perfusor Space
Summary of Contents for Braun Perfusor Space
-
Page 1
Perfusor® Space and Accessories Instructions for Use It is recommended that all pumps at your care unit are equipped with the same software version. Valid for software 688M… -
Page 2: Table Of Contents
CONTENTS Perfusor® Space Overview………………………3 Symbols on Product ……………………….5 Patient Safety ……………………….6 Menu Structure / Navigation……………………11 Chapter 1 Operation ……………………..14 1.1 Start of Infusion …………………….14 1.2 Entry With Different Combinations of Rate, VTBI (= Volume To Be Infused) and Time……………………….15 1.3 Bolus Application ……………………16 1.4 Syringe Change and New Therapy Start …………….17 1.5 End of Infusion……………………..18 1.6 Standby Mode ……………………..18…
-
Page 3: Perfusor® Space Overview
PERFUSOR SPACE® OVERVIEW P E R F U S O R ® S PA C E O V E R V I E W Arrow up and -down Press to reset single values Drive head with Scroll through menus, change setting of numbers from 0-9,…
-
Page 4
For vertical fixation of PoleClamp push lever down and rotate either way until lever clicks into notch. Push lever for rotation. Caution: A maximum of three B. Braun Space pumps can be stacked together when used with the PoleClamp SP. -
Page 5: Symbols On Product
SYMBOLS ON PRODUCT S Y M B O L S O N P R O D U C T Symbol Explanation Mandatory action: see instruction for use. See accompanying documents. Type CF unit with defibrillation protection Protection class II device Symbol indicating separate collection for electrical and electronic equipment (2002/96/EC) CE mark compliant to Directive 93/42/EEC…
-
Page 6: Patient Safety
Operation • The initial training of the Perfusor® Space is to be performed by B. Braun sales personnel or other authorized persons. After each software update, the user is required to inform himself of the changes to the device and accessories by referring to the Instructions for Use.
-
Page 7
PATIENT SAFETY • Only connect to patient once the syringe has been inserted correctly and there is proper fixation of the syringe pressure plate by the claws of the drive head. Interrupt connection during syringe change to prevent incorrect dose delivery. -
Page 8
PATIENT SAFETY Enteral Nutrition The Perfusor® Space may be used for enteral nutrition. Do not use enteral fluids for intravenous infusion as this may harm your patient. For this reason only use disposables dedicated and labeled for enteral nutrition. Other components •… -
Page 9
TCI and properly trained in using the present device. • The use of TCI with B. Braun Space does not limit the responsibility of the anaesthetist for administration of drugs. They need to be fully aware of the available literature for any parameter set used in association with a drug and need to refer to the prescribed information for rate and dosing limits. -
Page 10
PATIENT SAFETY • While using TCI an appropriate patient monitoring is mandatory. • Take care of using the right dilution/concentration of the drug and make sure the right dilution is selected at the pump. • Never administer Propofol or Remifentanil by a second infusion as long as you use TCI. -
Page 11: Menu Structure / Navigation
MENU STRUCTURE / NAVIGATION MENU STRUCTURE / NAVIGATION Cutline On/Off button OK button Start/Stop button Keypad with arrow up, -down, -left, -right button Bolus button Connection button Clear button All display screen shots are examples and may be different when related to an individual patient and individualized therapy.
-
Page 12
MENU STRUCTURE / NAVIGATION Display Meaning All status information is available in the bottom line of the dislplay. The desired information can be selected by using and will be displayed permanently thereafter (e. g. drug long name, time until syringe empty, current system pressure etc.). -
Page 13
MENU STRUCTURE / NAVIGATION Start Up Main Special Options Status Menu Menu Functions Menu Menu Syringe Dose Rate Occlusion Intermediate Dose selection Calculation Pressure volume Intermediate Prime ? Concentration Drug Library OccluGuard amount Change-over Use last Pressure Intermediate Weight therapy ? from Leap/Drop time… -
Page 14: Chapter 1 Operation
OPERATION Chapter 1 OPERATION 1.1 Start of Infusion • Ensure correct installation of the pump device. If the pump is connected to mains, the display states information such as the battery status, the mains connection symbol and the last therapy. •…
-
Page 15: Entry With Different Combinations Of Rate, Vtbi (= Volume To Be Infused) And Time
OPERATION Chapter 1 • Press to commence infusion. Running arrows on display and green LED above display indicate pump is infusing. Note: Stop the infusion at any time by pressing . The pump can be turned off at any time by pressing for 3 sec (Exception: Data lock level 2) and as long a disposable is inserted.
-
Page 16: Bolus Application
OPERATION Chapter 1 a) Target symbol is placed in front of VTBI: • Change of VTBI => Adjustment of time. Old and new target: VTBI • Change of time => Adjustment of rate. Old and new target: VTBI b) Target symbol is placed in front of time: •…
-
Page 17: Syringe Change And New Therapy Start
OPERATION Chapter 1 1.4 Syringe Change and New Therapy Start Note: To avoid incorrect dosing, always disconnect the pump from the patient when changing the syringe. Never leave the pump device unattended during syringe change. Before inserting a new syringe check if the axial fixation is properly working. •…
-
Page 18: Standby Mode
OPERATION Chapter 1 • Open pump cover. Remove the syringe, move the syringe holder into an upright position and close the front door. • Press for 3 sec. to switch the pump off. The drive moves into parking position. Note: The settings will be permanently saved by the switched off device.
-
Page 19: Chapter 2 Advanced Operations
OPERATION Chapter 1 ADVANCED OPERATIONS 2.1 Status Request of Pump when Infusion is Running Press to switch between run display and Main Menu while the device is infusing. Navigate through the menu using to check parameters. In order to check the menu parameters in the Status-/Options Menu, select «Status»…
-
Page 20: Chapter 3 Special Functions
ADVANCED OPERATIONS Chapter 2 SPECIAL FUNCTIONS 3.1 Dosing Units and Dose Rate Calculation (Overview) The following list shows the units used in the pump: Gram family: ng, mcg, mg, g Unit family: mIU, IU, kIU, MIU Equivalents family: mEq Mole family: mmol Kilocalorie family: kcal Millitliter family:…
-
Page 21: Dose Rate Calculation (Operation)
SPECIAL FUNCTIONS Chapter 3 3.2 Dose Rate Calculation (Operation) a Select dose rate calculation with l. a Select the unit of the active ingredient with and confirm it with l. a Enter the concentration by entering the amount of the active ingredient and the volume.
-
Page 22
SPECIAL FUNCTIONS Chapter 3 On the one hand, a drug name including the according therapy data can be taken from the drug library. On the other hand, if a rate, VTBI and/or time were already defined in the Main Menu, the drug name and the adjusted values of the data set will be loaded. -
Page 23
SPECIAL FUNCTIONS Chapter 3 • Select the desired drug with and press l. Before the initial bolus begins, the bolus menu is displayed to allow editing the bolus with q. • Check the parameter and start infusion with Hard Limits: If the set rate/dose/bolus volume and bolus rate exceed the values stored in the drug library (hard limits), the drug will be rejected, a hint will be displayed and the pump will fall back into the drug selection. -
Page 24: Patient Controlled Analgesia (Pca) (Optional)
SPECIAL FUNCTIONS Chapter 3 The Drug Library Upload starts as soon as the pump is in Passive mode. You can cancel the upload by pressing c. Note: Please contact your local sales represantative in case you like to use Remote Drug Library update.
-
Page 25
SPECIAL FUNCTIONS Chapter 3 In this state the patient is allowed to demand boli. Depending on the status of the therapy these are either administered or denied. Changing the syringe is also possible by using the code for level 1 or level 2. Altering the settings for PCA or other therapies however is only possible with the code for level 3. -
Page 26: Target Controlled Infusion (Tci) (Optional)
The pharmacokinetic model and its parameters are schematically depicted by the following illustration: B. Braun Space is offering two modes for TCI: • TCI by targeting the plasma concentration In this mode the user selects the desired concentration of a drug in the…
-
Page 27
TCI. A pharmacokinetic model modi- fied in such way is schematically depicted by the illustration on the next page. TCI with B. Braun Space is possible with two drugs: Propofol and Remifentanil. For Propofol the user can choose between two parameter sets. The parameter… -
Page 28
SPECIAL FUNCTIONS Chapter 3 Drug / Parameter Propofol Remifentanil [Litre] 0,228 * Weight 4,27 5,1 — 0,0201 * (Age — 40) + 0,072 * (LBM — 55) [min 0,119 0,443 + 0,0107 * (Weight — [2,6 — 0,0162 * (Age — 40) + 77) — 0,0159 * (LBM — 59) + 0,0191 * (LBM — 55)] / [5.1 — 0,0062 * (Height — 177) -
Page 29
Chapter 3 Important note: Before installing an additional drug list please contact your local B. Braun representative! Setting up the pump For TCI a drug list with at least one drug activating the profile TCI is necessary. The drug list in this version is pre-defined. By this the conditions for an effective and safe therapy are defined. -
Page 30
SPECIAL FUNCTIONS Chapter 3 Important notes: • Be sure to enter the data corresponding to the respective patient. • Once the TCI is started patient data can not be altered! Editing a target and starting TCI The editor window for setting the target comes up with the default value from the drug list. -
Page 31
SPECIAL FUNCTIONS Chapter 3 Useful information while pump is running By pressing additional information can be requested. Pressing a second time is offering a graphical overview. The line describes the course of Cp over the time and the area describes the course of Ce over the time. -
Page 32: Barcoding
SPECIAL FUNCTIONS Chapter 3 Barcoding The barcoding functionality is included but initially not activated. Please contact your local sales representative in case you like to use barcoding. Ramp and Taper Mode The Ramp and Taper Mode is designed to deliver infusions with gradual ramp up and taper down rates.
-
Page 33
SPECIAL FUNCTIONS Chapter 3 Set Profile Parameters: The therapy can be started directly via the drug library or via the Main Menu/Special functions. Starting Ramp and Taper via Drug Library: Note: Ramp and Taper settings have been configured in the Drug List Manager before and have been uploaded into the pump. -
Page 34: Program Mode
SPECIAL FUNCTIONS Chapter 3 Taper phase The pump linearly decreases the rate in the predefined time until it reaches the KVO rate Note: After starting infusion it is only possible to change rates, time and VTBI in the continuous phase. By editing (increasing/decreasing) the plateau rate, the therapy is recalculated.
-
Page 35
SPECIAL FUNCTIONS Chapter 3 Example: Program Mode should only be performed by an experienced user being familiar with the principles of the Program Mode function and properly trained in using the present device. Note: The active Program Mode function always displays this icon in the Display Note: Bolus function is disabled for Program Mode. -
Page 36: Intermittent Mode
SPECIAL FUNCTIONS Chapter 3 The pump can be started now by pressing Starting Program Mode via Special Function Menu: • Switch on pump with and wait until self-check is finished. • Insert disposable. • Go to Special Functions Menu and select Program Mode. •…
-
Page 37
SPECIAL FUNCTIONS Chapter 3 Example: Intermittent Mode should only be performed by an experienced user being familiar with the principles of the Intermittent Mode and properly trained in using the present device. Note: The active Multi Dose Mode function always displays this icon in the Display Note: Regular Bolus function is disabled for Intermittent Mode. -
Page 38
SPECIAL FUNCTIONS Chapter 3 Starting Intermittent Mode via Special Function Menu: • Switch on pump with and wait until self-check is finished. • Insert disposable. • Go to Special Functions Menu and select Intermittent Mode. • Press to enter parameters and to confirm. -
Page 39: Dose Over Time
Note: The feature Dose Over Time always requires the usage of dosing units (i.e., mg or mg/kg patient weight). Before using Dose Over Time contact your local B. Braun representative! Starting Dose Over Time via Drug Library: Note: Dose Over Time settings have been configured in the Drug List Manager before and have been uploaded into the pump.
-
Page 40
SPECIAL FUNCTIONS Chapter 3 • Insert disposable and use the drug library according to the Instructions for Use. • Select a drug by using and press l. The pump now offers the possible therapy profiles. Select “Dose over Time” with press l. -
Page 41: Take Over Mode (Tom) (Optional)
SPECIAL FUNCTIONS Chapter 3 3.11 Take Over Mode (TOM) Take Over Mode is a feature to support the user during syringe changes by auto- matically starting a second Perfusor® Space pump when the first has run empty. The second pump automatically takes over the infusion rate from the first pump. Activation: •…
-
Page 42
SPECIAL FUNCTIONS Chapter 3 • Navigate through the list with and select in alphabetical order (all drugs) or within a category with l. The drug selected in the second pump must be the same as the first. • Navigate through the list with and select a concentration with l. -
Page 43
SPECIAL FUNCTIONS Chapter 3 Note: Start-up behaviour is not influenced by TOM. See Chapter Start Up Graphs and Trumpet Curves. Note: Please use a seperate patient connection for Take Over Mode infusion (e.g. smallbore extension set) or use a back check valve for lines at the same access which are not used for Take Over Mode. -
Page 44
SPECIAL FUNCTIONS Chapter 3 Recommendation Ensure first Perfusor® Space pump is infusing Ensure first Perfusor® Space pump must be running in ‘continuous mode’ (i.e. ml/h or a dose rate; not KVO, PCA etc.) Deactivate Data Lock Data connection must be active between pumps –… -
Page 45
SPECIAL FUNCTIONS Chapter 3 Changes in TOM system: Change Reaction Rate changed in pump No user interaction necessary, will start infusion at new rate when syringe is empty. pump is stopped pump shows “connection lost – TOM aborted” alarm. TOM may be reactivated by pump is put in standby pressing and then… -
Page 46: Chapter 4 Autoprogramming
AUTOPROGRAMMING Chapter 4 A U TO P R O G R A M M I N G Note: All normal pump functions remain in place when orders are received via autoprogramming. The pump can accept drug orders wirelessly from the EHR system or from SpaceStation with SpaceCom.
-
Page 47
AUTOPROGRAMMING Chapter 4 Note: Order may be cancelled prior to confirming order. • Once all values are confirmed, the Main Menu is displayed. Note: Soft Limit alert will be issued if value exceeds any soft limits set in drug library, soft limit may be overridden or value re-programmed per institutional policy. -
Page 48
AUTOPROGRAMMNG Chapter 4 • Follow prompt, pressing to accept order or key to cancel and hold order for later. New Primary Infusion: • To accept a new PRIMary order, stop infusion and clear current PRIMary infusion by pressing key and responding “yes” to clear current infusion. PIGGYback Orders: Orders received after PRIMary has been set will be for PIGGYback infusions, follow prompts on screen to stop the PRIMary to accept the PIGGYback order. -
Page 49
AUTOPROGRAMMING Chapter 4 Note: Changing values on any incoming order may only be done after confir- ming all values. Once all values are confirmed you may scroll to any value and open editor with to change value. Alternately, order may be cancelled and request made for revised order to be sent. -
Page 50: Chapter 5 Options
OPTIONS Chapter 5 OPTIONS The options functions may be selected and changed while the pump is infusing or stopped. To edit a menu item, select “Options” in the Main Menu and press l. Then select desired function with and follow the Instructions for Use as described. 5.1 Occlusion Pressure The higher the pressure level is set at, the higher the pressure level must rise before triggering an occlusion pressure alarm.
-
Page 51
OPTIONS Chapter 5 OccluGuard activation / deactivation from the Main Menu • Go to Options Menu and press l. • Navigate through the list with and select OccluGuard. • OccluGuard can be activated with and deactivated with d. Pressure Leap/Drop detection The pressure leap/drop software detects sudden increases and decreases in infu- sion pressure respectively which can be caused by problems in IV access, or changes in pump position in the SpaceStation. -
Page 52
OPTIONS Chapter 5 OccluGuard Meaning Recommendation Symbol OccluGuard is active. Infusion is running stably Pending – OccluGuard has not enough data OccluGuard will automatically reactivate as soon as infusion rate OccluGuard Inactive drops below threshold levels – see above. Confirm alarm and check IV access, IV setup and syringe for cause of Occlusion has been occlusion. -
Page 53: Data Lock
OPTIONS Chapter 5 When a change is made to the infusion system (e.g. addition or removal of a pump to a SpaceStation, a change of infusion rate, a bolus application) the OccluGuard and pressure leap/drop are temporarily set to ‘pending’ ( ) to allow the system to reach a hydrostatic balance, and so prevent false alarms.
-
Page 54: Bolus Rate
OPTIONS Chapter 5 Activation of the function: • Open data lock in Options Menu with l. • Select between level 1, 2 or 3 (if activated) with and confirm with k. • Enter code with and press in order to activate data lock. Changes to the protected values and the bolus function which are marked withy are only possible after entering the code.
-
Page 55: Alarm Volume
OPTIONS Chapter 5 5.7 Alarm Volume Chose between 9 different alarm volume levels. • Open alarm volume in Options Menu with l. • Set volume with and confirm entry with k. 5.8 Date / Time • Open date/time in Options Menu with l. •…
-
Page 56: Chapter6 Alarms
ALARMS Chapter 5 ALARMS The Perfusor® Space is equipped with a audible and optical alarm signal. Audible Alarm- Optical signal Staff call User confirmation type signal Red LED Yellow LED Text Device flashes device Press and follow Alarm alarm and the instruction on the alarm code display.
-
Page 57
ALARMS Chapter 6 Display message Pre-alarm reason “Syringe nearly empty“ Very little fluid is left in syringe. “VTBI near end“ The preselected volume is nearly infused. “Time near end“ The preselected time is almost over. “Battery nearly empty“ The battery is almost discharged. “KVO mode“… -
Page 58
ALARMS Chapter 6 the alarm message, and the staff call (optional) are all cleared by pressing k. Corrections should be made in accordance with the alarm reason. Display message Alarm reason “Syringe empty“ There is no fluid left in the syringe. Due to varying syringe tolerances of syringes from other manufacturers, some fluid may be left inside the syringe. -
Page 59
ALARMS Chapter 6 “Calibrate device“ Pump calibration data have changed (e.g. after an update). Recalibrate device via the service program. “Claw malfunction“ The emergency release button was pressed and the claws manually opened. Take out syringe and contact technical service department. “Plunger plate not prop. -
Page 60: Reminder Alarms
ALARMS Chapter 6 6.3 Reminder Alarms Reminder alarms only occur in two cases: 1. A syringe is inserted, the pump doesn’t administrate, no value is being edited and the device is not operated for two minutes. An acoustic tone sounds, the yellow LED is constantly on and a staff call is activated.
-
Page 61: Battery Operation And Maintenance
BATTERY OPERATION AND MAINTENANCE Chapter 7 BATTERY OPERATION AND MAINTENANCE The battery has an operating lifetime of 8 hours at 25 ml/h when new. For optimal treatment of the battery, the device is equipped with protection against overcharge and deep depletion. The battery pack is charged by the pump during connection to mains.
-
Page 62
BATTERY OPERATION AND MAINTENANCE Chapter 7 • If a battery, which is not completely discharged, is charged several times, its capacity can be reduced. • Under normal temperature conditions a battery can be charged and discharged approx. 500 times before its lifetime decreases. •… -
Page 63: Chapter 8 Compatible Syringes
) to ensure specific syringe brand compatibility. The Time to Occlusion alarm has been measured at 5 ml/h. The measured data are typical values which may vary because of possible syringe tolerances. Manufacturer: B. Braun Syringe Type Omnifix Omnifix Omnifix Omnifix Omnifix B.
-
Page 64
COMPATIBLE SYRINGES Chapter 8 Manufacturer: TYCO USA Syringe Type Monoject Monoject Monoject Monoject Monoject Monoject 3 ml 6 ml 12 ml 20 ml 35 ml 50/60 ml TYCO USA Mat. No. 8881- 8881- 8881- 8881- 8881- 8881- 513934 516937 512878 520657 535762 560125… -
Page 65
COMPATIBLE SYRINGES Chapter 8 Manufacturer: TERUMO Syringe Type 3 ml 5 ml 10 ml 20 ml 30 ml 50 ml 60 ml TERUMO EU/USA/JAP Mat. No. 3SS*03L 3SS*05L 3SS*10L 3SS*20L 1SS*30LZ1 2BS-50LG 3SS*60L 1SS*05LZ1 1SS*10LZ1 SS*20ES typ. typ. typ. typ. typ. -
Page 66
COMPATIBLE SYRINGES Chapter 8 Manufacturer: Becton-Dickinson Syringe Type BD Precise BD Precise B-D Precise 50 ml A/P 20 ml A/P Mat. No. 300144 300141 Time to Occl. [mm:ss] 03:17 01:11 [mm:ss] 16:36 05:03 Manufacturer: Polfa Syringe Type Polfa 50 ml Mat. -
Page 67
COMPATIBLE SYRINGES Chapter 8 Syringes not specified in IEC/EN 60601-2-24 Nutrition pumps, in contrast to infusion pumps, are not classified as Class IIa according to the infusion pump norm IEC/EN 60601-2-24. There are therefore no direct guide- lines concerning the technical characteristics (accuracy of infusion rate, alarm parameters etc) of the relevant disposables. -
Page 68: Start Up Graphs And Trumpet Curves
START UP GRAPHS AND TRUMPET CURVES Chapter 9 START UP GRAPHS AND TRUMPET CURVES Start Up Curves Trumpet Curves The graphs show the accuracy/uniformity of flow in relation to time. They allow for the following: The delivery behaviour or delivery precision is essentially influenced by the type of (disposable syringe) used.
-
Page 69: Is Depending On Chapter10 Technical Data
Moisture protection IP 22 (fluid protected for horizontal usage) External power supply: • Rated voltage Via B. Braun SpaceStation or optional mains adaptor (rated voltage 100 … 240 V AC~, 50/60 Hz) for stand alone operation • External low voltage 11 ……
-
Page 70
Delivery rate 1 ml/h: KVO-rate = set rate (default setting 0.1 ml/h) Computer connection USB connection in combination with B. Braun interface lead CAN SP (8713230) including electrical insulation. Please pay attention to safety notices. History protocol < 3000 last history entries. -
Page 71
TECHNICAL DATA Chapter 10 • Only use combined with approved devices/accessories by the manufacturer, otherwise this may lead to higher emission or reduced immunity. • Use only compatible combinations of equipment, accessories, working parts and disposables with luer lock connectors. Essential Performance for Infusion pumps: •… -
Page 72
TECHNICAL DATA Chapter 10 EMC (ELECTROMAGNETIC COMPATIBILITY) -
Page 73
TECHNICAL DATA Chapter 10… -
Page 74
TECHNICAL DATA Chapter 10… -
Page 75
TECHNICAL DATA Chapter 10… -
Page 76: Warranty / Tsc* / Service / Training / Cleaning / Disposal
• the Technical Safety Checks are carried out regularly. Warranty The CE mark confirms that this B. Braun provides 24 months warranty, as from the date of delivery, for every medical product Perfusor® Space (12 months for every Battery-Pack SP). This covers repair or complies with the replacement of parts damaged as a result of design/manufacturing errors or «Council Directive…
-
Page 77
Note: Do not use Hexaquart® or other alkylamine containing disinfectants. Recommended: disinfectant for wiping available from B. Braun: Meliseptol® Foam pure, Melsitt 10% and Melsept SF 10%. Note: Keep instrument upright and do not allow any part of instrument to become saturated with or submersed in fluid during cleaning operation. -
Page 78
Disposal The pumps as well as battery packs can be returned to B. Braun for further disposal. When taking care of disposing of disposables as well as infusion soluti- ons, please consider the applicable hygiene and disposal regulations. -
Page 79: Instructions For Use Accessory
Comfort additionaly includes a central alarm management and alarm LEDs. PoleClamp SP (8713130) A maximum of three B. Braun Space pumps and one SpaceControl can be stacked together when used with the PoleClamp SP. For detailed instructions on secure fixation of the PoleClamp SP please refer to «Overview Perfusor® Space»…
-
Page 80
INSTRUCTIONS FOR USE ACCESSORY Chapter 12 3.) Push plug of Connection Lead SP into 12 V connector. Note: A maximum of three plugs can be stacked upon each other in socket P2. Battery-Pack SP (NiMH) (8713180) Battery-Pack SP (NiMH) incl. Pin (8713180A) For further information on the Battery-Pack SP (NiMH) see “Battery Operation”. -
Page 81
INSTRUCTIONS FOR USE ACCESSORY Chapter 12 Connection Lead SP (12 V) (8713231) Install the Connection Lead SP (12 V) in the following way: 1.) Connect plug to socket P2 on back of pump or F3 on SpaceStation respectively. 2.) Put the connection lead into the car socket. 3.) If necessary, remove red adaptor of motor vehicle connector by slightly turning and simultanously pulling. -
Page 82
INSTRUCTIONS FOR USE ACCESSORY Chapter 12 Caution: The user should always closely observe the local pump alarms as well. Note: A maximum of three plugs can be stacked upon each other in socket P2. Technical Data Connecting Wire white and green white and brown Alarm disconnected… -
Page 83
INSTRUCTIONS FOR USE ACCESSORY Chapter 12 P C A — A c c e s s o r i e s • Space PCA-Kit (REF 8713554) consisting of: : — Demand button — Hook and loop tape for fixation of the demand button at the patient s arm — Line fixation connection between hook and loop tape… -
Page 84: Ordering
ORDERING Art. No. B. Braun Perfusor® Space (100 – 240 V) ……….871 3030 Recommended accessories for the B. Braun Perfusor® Space: SpaceStation ………………..871 3140 SpaceCover Standard ………………871 3147 SpaceCover Comfort ………………871 3145 PoleClamp SP…………………871 3130 Power Supply SP EU III ……………….871 3110D Power Supply SP EU III 3.0m……………..871 3123D…
-
Page 85
ORDERING 50ml, yellow inked cylinder and aspiration needle…….87 28801 F 50ml, yellow inked cylinder, aspiration needle and 15 µm particle filter …………….872 8800 F 50ml, black, aspiration needle and particle filter……..872 8828 F Omnifix® syringes Omnifix® 50/60 ml Luer Lock……………461 7509F Omnifix®… -
Page 86
ORDERING Type PCA, PVC tube 1,5 mm, 150 cm, Luer Lock ……..872 6019 with 0.2 µm Sterifix filter, PVC tube 1,5 mm, 200cm, Luer Lock …………………872 3001 with SafeSite valve, PVC tube 1,5 mm, 150 cm, Luer Lock………………..872 2820… -
Page 90
Manufactured by: B. Braun Melsungen AG B. Braun Melsungen AG Sparte Hospital Care 34209 Melsungen 34209 Melsungen Germany Germany Tel +49 (0) 56 61 71-0 Tel.: +49 (0) 56 61 71-0 Fax: +49 (0) 56 61 71-20 44 38916517 • Drawing No. I0688700201 Printed on pulp bleached 100 % chlorine-free www.bbraun.com…

Perfusor® Space
Service Manual
Version 1.3 English

0
|
This Service Manual is valid for: |
Designation |
Part No. |
|
Infusion syringe pump Perfusor® Space . . . . . . . . . . . |
. 0871 3030 |
|
|
This Service Manual is available under |
Designation |
Part No. |
|
the following part number: |
Service Manual Perfusor® Space, English. . . . . . . . . . . |
8713 9020 |
|
Languages of this Manual |
The Service Manual for this unit can be supplied in the following |
|
|
languages: |
||
|
Designation |
Part No. |
|
|
Service Manual Perfusor® Space, German . . . . . . . . . . |
8713 9010 |
|
|
Service Manual Perfusor® Space, English (US). . . . . . |
8713 9020U |
|
|
Service Manual Perfusor® Space, French . . . . . . . . . . . |
8713 9030 |
|
2 |
Perfusor® Space, 1.2 gb |

Table of Contents 0
|
Important Preliminary Remarks |
Service Work |
Page |
0 — 5 |
|
Technical Safety Checks |
Page |
0 — 5 |
|
|
Current Versions |
Page |
0 — 5 |
|
|
Revision Service |
Page |
0 — 5 |
|
|
Quality Management |
Page |
0 — 6 |
|
|
Checks and Repair |
Page |
0 — 6 |
|
|
Notes on ESD |
Page |
0 — 6 |
|
|
Spare Parts and Test Equipment |
Page |
0 — 7 |
|
|
Setting Off |
Page |
0 — 7 |
|
|
Contact Persons |
Technical Training |
Page |
0 — 11 |
|
Entry for Technical Training |
Page |
0 — 11 |
|
|
Ordering of Spare Parts and Test Equipment |
Page |
0 — 11 |
|
|
Service Hotline International |
Page |
0 — 11 |
|
|
Return of Spare Parts and Test Equipment |
Page |
0 — 11 |
|
|
Safety Officer |
|||
|
(§ 30 MPG) |
Page |
0 — 11 |
|
|
Translation |
Page |
0 — 11 |
|
|
System Overview |
Description |
Page |
1 — 1 |
|
System Overview |
Page |
1 — 1 |
|
|
Physical Construction |
Page |
1 — 2 |
|
|
Function |
Page |
1 — 3 |
|
|
Unit Software |
Page |
1 — 6 |
|
|
Service Program |
Page |
1 — 7 |
|
|
Technical Data |
Page |
1 — 12 |
|
|
Options |
Page |
1 — 12 |
|
|
Accessories |
Page |
1 — 12 |
|
|
Unit Diagnosis / Calibration |
General |
Page |
2 — 1 |
|
Alarms and Error Codes |
Page |
2 — 3 |
|
|
The Most Important Error Modes |
Page |
2 — 8 |
|
|
Device Check |
Page |
2 — 9 |
|
|
Calibration |
Page |
2 — 14 |
|
|
Procedural Instructions for Calibration |
Page |
2 — 14 |
|
|
Trouble Shooting |
Page |
2 — 29 |
|
|
Disassembly / Assembly |
General |
Page |
3 — 1 |
|
Battery Module |
Page |
3 — 9 |
|
|
Unit Foot |
Page |
3 — 11 |
|
|
Operating Unit |
Page |
3 — 12 |
|
|
Upper Part of Housing |
Page |
3 — 17 |
|
|
Release Button |
Page |
3 — 19 |
|
|
Loudspeaker |
Page |
3 — 19 |
|
|
Drive |
Page |
3 — 20 |
|
|
Syringe Holder with Piston Brake |
Page |
3 — 27 |
|
|
Processor PCB |
Page |
3 — 34 |
|
|
Assembly / Installation |
Page |
3 — 35 |
|
|
Checks after Repair |
Page |
3 — 47 |
|
Perfusor® Space, 1.0 gb |
0 — 3 |

|
Servicing the Unit |
Cleaning |
Page |
4 |
— 1 |
|
Servicing the Battery |
Page |
4 |
— 1 |
|
|
Technical Safety Check (TSC) |
Perfusor® Space 1 |
|||
|
Technical Safety Check (TSC) |
Power Supply SP 1 |
|||
|
Procedural Instructions on the TSC |
Visual Inspection |
Page |
7 |
— 1 |
|
Electrical Safety |
||||
|
according to IEC/EN 60601-1 |
||||
|
or VDE 0750 and VDE 0751 |
Page |
7 |
— 2 |
|
|
Functional Inspection Perfusor® Space |
Page |
7 |
— 3 |
|
|
Functional Inspection Power Supply SP |
Page |
7 |
— 6 |
|
|
Test Equipment and Special Tools |
Test equipment |
Page |
8 |
— 1 |
|
Special Tools |
Page |
8 |
— 3 |
|
|
Spare Parts List |
Page |
9 |
— 1 |
|
|
Revision Documentation |
Description of Version |
Page |
10 |
— 1 |
|
Version List of the Individual Pages |
Page |
10 |
— 1 |
|
|
Index |
Page |
11 |
— 1 |
|
0 — 4 |
Perfusor® Space, 1.0 gb |

Important Preliminary Remarks 0
Service Work |
The present manual is for your information only. The possession of |
|
this manual does not authorize the performance of service work. |
|
|
Service tasks may only be executed by persons, who |
|
|
— have received appropriate training on the system from |
|
|
B. Braun |
|
|
— are included in the revision service |
|
|
— possess the necessary test equipment and mechanical aids, |
|
|
and |
|
|
— fulfill the personal requirements (training and knowledge). |
|
Technical Safety Checks |
The user is obliged to perform or to have performed the Technical |
|
Safety Checks on those medial products for which these checks |
|
|
have been prescribed by the manufacturer and to carry them out |
|
|
according to the indications of the manufacturer as well as the |
|
|
generally approved technical standards while adhering to the |
|
|
periods stated (§ 6 MP BetreibV). |
|
|
B. Braun also recommends training on the Technical Safety |
|
|
Checks, or to perform at least the steps indicated in the current |
|
|
version of the manual, as: |
|
|
— the TSC requires that the instructions in the manuals are |
|
|
observed |
|
|
— the manuals are a reference for measurements |
|
|
— depending on the unit type, the Service Program must be |
|
|
called which may lead to a dangerous unit condition in case |
|
|
of inappropriate operation. Furthermore, a special service |
|
|
connector may be necessary. |
|
Current Versions |
This manual version corresponds to the state when the manual |
|
was written. B Braun reserves the right to make technical |
|
|
modifications. The state of the revision is indicated by the index |
|
|
number in the footer of every page. |
|
Revision Service |
The possession of this manual does not automatically mean |
|
inclusion in the revision service. You will be included in the |
|
|
revision service after: |
|
|
— technical training by B. Braun Melsungen or |
|
|
— a written order placed with the sales department of B. Braun |
|
|
(fee required). |
|
Perfusor® Space, 1.1 gb |
0 — 5 |

|
0 |
Important Preliminary Remarks |
|
Responsibility of the Manufacturer |
The manufacturer, person who assembles, installs or imports the |
|
device can only be held responsible for safety, reliability and |
|
|
performance if |
|
|
— mounting, enhancements, new settings, changes or repairs |
|
|
are carried out by duly authorized persons, |
|
|
— the electrical installation in the corresponding room meets |
|
|
the requirements of the VDE 0107, VDE 0100 part 710 or |
|
|
IEC 60364-7-710 and the national standards, |
|
|
— the device is used in accordance with the instructions for use |
|
|
and the Service Manual, |
|
|
— the Technical Safety Checks are performed at regular |
|
|
intervals, |
|
|
— a current manual which corresponds to the revision state is |
|
|
used when carrying out maintenance, repair and service, |
|
|
— the service technician takes part in the revision service, |
|
|
— the technician has participated in a technical training course |
|
|
for the specific B. Braun unit. |
|
Quality Management |
B. Braun is certified in accordance with DIN EN ISO 9001 and |
|
ISO 13485. This certification also includes maintenance and |
|
|
service. |
|
|
The unit has the CE label. The CE label confirms that the device |
|
|
corresponds to the “Directive of the Council for Medical Products |
|
|
93/42/EC” of June 14, 1993. |
|
Checks and Repair |
Training may only be performed by B. Braun. The possession of the |
|
manual does not authorize the performance of repairs. The |
|
|
instructions on electrostatic sensitive components (ESD |
|
|
standards) must be observed. |
|
|
After repair a device check or diagnosis is to be carried out. |
|
Notes on ESD |
Semiconductors can be destroyed by electrostatic discharge. |
|
Especially MOS components can be damaged by interference from |
|
|
electrostatic fields, even without discharge via contact. This type |
|
|
of damage is not immediately recognizable. Unit malfunctions |
|
|
can even occur after a longer period of operation. |
|
0 — 6 |
Perfusor® Space, 1.1 gb |

|
Important Preliminary Remarks |
0 |
Fig.: 0 — 1
Spare Parts and Test Equipment
Setting Off
Each workstation must be equipped according to the recommendations with the necessary static protective measures, if ESD components or boards are handled.
Each workstation must be equipped with a conductive table surface. The conductive surface, the soldering iron or the soldering stations must be grounded via protective resistors.
Chairs must be of antistatic design. The floor or floor mats should be of electrically conductive material.
Personnel must wear conductive wristbands which are connected to a central ground potential via protective resistors, e.g. the ground contact of a wall outlet. Furthermore it is recommended that personnel wear cotton clothing and electrically conductive shoes to prevent electrostatic charge.
Only use original spare parts from the manufacturer. Do not tamper with assembly groups which can only be exchanged completely. The spare parts required are listed in the repair descriptions.
Service personnel are responsible for the calibration of their test equipment. Original test equipment can be calibrated at the works of B. Braun. Further information is available upon request.
Additional notes and warnings are set off as follows:
Note
Is used for additional or special notes concerning information and working steps.
CAUTION
Is used for working steps which may result in damage to the unit, system or to a connected device.
WARNING
IS USED FOR WORKING STEPS WHICH MAY RESULT IN PERSONAL INJURY.
|
Perfusor® Space, 1.1 gb |
0 — 7 |

|
0 |
Important Preliminary Remarks |
References to chapters are shown as follows (see “Setting Off“ pg. 0 — 7)
References to figures and tables are shown as follows
Fig.: 2 — 3 or Table 2 — 1
References to item numbers in figures are shown as follows (Fig.: 1 — 1 / Item 1)
In this case “Fig.: 1 — 1“ is the figure number and “Item 1“ the item number within the figure.
When the Service Manual is stored as pdf-file, these references are displayed green. Click with the mouse button on a reference to jump to the corresponding source.
Menu commands are described as: Menu File.
|
0 — 8 |
Perfusor® Space, 1.1 gb |

|
Important Preliminary Remarks |
0 |
|
List of Abbreviations |
Abbreviations which are not generally known, but are used in this |
|
|
manual, are listed below. |
||
|
CAN |
Controller Area Network |
|
|
CE |
Communauté Européenne |
|
|
(European Communities) |
||
|
CS |
Calibration Step |
|
|
DIN |
Deutsche Industrie Norm |
|
|
(German Industrial Standard) |
||
|
EN |
European Standard |
|
|
ESD |
Electrostatic Discharge |
|
|
FuP |
Function Microprocessor |
|
|
IEC |
International Electrotechnical |
|
|
Commission |
||
|
ISO |
International Standardization |
|
|
Organization |
||
|
ISP |
Infusomat® Space |
|
|
ISPS |
Infusomat® Space, Silicon |
|
|
ISPP |
Infusomat® Space, PVC |
|
|
KuP |
Monitoring Microprocessor |
|
|
LCD |
Liquid Crystal Display |
|
|
MOS |
Short for the following |
|
|
company name: |
||
|
MOS Technology, Inc. |
||
|
(Commodore Semiconductor |
||
|
Group) |
||
|
PCA |
Patient-Controlled Analgesia |
|
|
PSP |
Perfusor® Space |
|
|
SP |
Space (System) |
|
|
SPC |
SpaceCover |
|
|
SPCC |
SpaceCover comfort |
|
|
SPCS |
SpaceCover standard |
|
|
SPCO |
SpaceCom |
|
|
SPCT |
SpaceControl |
|
|
SPS |
SpaceStation |
|
|
TEMP |
Temperature |
|
|
TS |
Troubleshooting Step |
|
|
TSC |
Technical Safety Checks |
|
Perfusor® Space, 1.2 gb |
0 — 9 |

|
0 |
Important Preliminary Remarks |
|
UTS |
Unit Test Step |
|
VDE |
Verband der Elektrotechnik, |
|
Elektronik und |
|
|
Informationstechnik e.V. |
|
|
(German electrical engineering |
|
|
association) |
|
0 — 10 |
Perfusor® Space, 1.2 gb |

Contact Persons 0
Technical Training |
Via local representative. |
||
Entry for Technical Training |
Application for a technical training course must be made via the |
||
|
responsible representative. |
|||
Ordering of Spare Parts and Test Equipment |
Please contact your local B. Braun subsidary. |
||
|
International Technicians (Intercompany) |
|||
|
Nadja Machal |
|||
|
Fax: |
+49 5661 / 75 — 47 89 |
||
|
e-mail: |
nadja.machal@bbraun.com |
||
Service Hotline International |
Karl Tippel, Tanja Kördel |
||
|
Fax: |
+49 5661 / 71 — 35 26 |
||
|
e-mail: |
karl.tippel@bbraun.com |
||
|
e-mail: |
tanja.koerdel@bbraun.com |
||
Return of Spare Parts and Test Equipment |
B. Braun Melsungen AG |
||
|
Schwarzenberger Weg 73-79 |
|||
|
Wareneingang Werk C |
|||
|
34 212 Melsungen |
|||
|
Germany |
|||
|
Safety Officer |
Dr. Ludwig Schütz |
||
|
(§ 30 MPG) |
e-mail: ludwig.schuetz@bbraun.com |
||
Translation |
Cs2 Informatik GmbH & Co. KG, Germany |
|
Perfusor® Space, 1.3 gb |
0 — 11 |

|
0 — 12 |
Perfusor® Space, 1.0 gb |

System Overview 1
Description |
The Perfusor® Space (PSP) is according to IEC/EN 60601 resp. IEC/ |
|
|
EN 60601-2-24 a transportable infusion syringe pump for |
||
|
administrating fluids in the nutritional therapy and infusion |
||
|
technique as well as for home care applications. |
||
|
The medical specialist must decide on suitability for application |
||
|
on the basis of the warranted properties and the technical data. |
||
System Overview |
The Space system is a modular design of modern infusion |
|
|
technology for stationary, mobile or private use. The key modules |
||
|
and their connection to the peripheral devices are shown in |
||
|
Fig.: 1 — 1. |
||
|
All the pump types, Perfusor® Space, Infusomat® Space and |
||
|
Infusomat® Space P, as well as the other devices of the system are |
||
|
1 |
||
|
of modular design. Up to three pumps can be connected together |
||
|
mechanically using L rails on the bottom of the unit and grooves |
||
|
2 |
on the top. They can then be fastened to a drip stand or |
|
|
appropriate rail using the pole clamp. |
||
|
The SpaceControl module can be used to extend operation. One |
||
|
3 |
single pump can be inserted onto this module. The pump is then |
|
|
connected via connectors to the module. |
||
|
The SpaceStation module allows the set-up of a complete pump |
||
|
system with up to 24 pumps. Up to four pumps can be installed in |
||
|
4 |
every SpaceStation. The pumps are supplied with power via the |
|
|
5 |
integrated power supply and the built-in connectors. The pumps |
|
|
are connected to the optional SpaceCom via these connectors. |
||
|
SpaceControl can also be integrated into the system. |
||
|
Up to six SpaceStations can be set-up as a column with a total of |
||
|
24 pumps. SpaceStation placed next to each other can be |
||
|
Fig.: 1 — 1 Space system |
connected via special connection cables, if the maximum number |
|
|
of 24 pumps in maximum three columns is not exceeded. |
||
|
Legend of fig. 1 — 1: |
SpaceCover Standard or SpaceCover Comfort forms the top of |
|
|
ItemDesignation |
||
|
each column. Alarms are signalled by a row of LEDs and a |
||
|
1 SpaceCover |
||
|
loudspeaker in the SpaceCover Comfort. |
||
2Infusion pump Infusomat® Space
3Infusion syringe pump Perfusor® Space
4SpaceControl
5SpaceStation
|
Perfusor® Space, 1.0 gb |
1 — 1 |

Physical Construction
5
4
3
 6
6
Fig.: 1 — 2 Perfusor® Space
Legend of fig. 1 — 2:
ItemDesignation
|
1 |
Perfusor® Space |
6 |
Connector “P2“ for SpaceStation module, external 12 V DC |
|
2 |
Drive head |
and accessories |
|
|
3 |
Syringe holder with piston brake |
7 Connector “P3“, connection to SpaceControl module |
|
|
4 |
Operating Unit |
8 |
Battery compartment cover |
|
5 |
Syringe area |
|
1 — 2 |
Perfusor® Space, 1.0 gb |

|
The Perfusor® Space housing mainly consists of the bottom part |
|
|
and the upper part. |
|
|
The battery module is inserted in the rear of the housing upper |
|
|
part. The opening is covered by the battery compartment cover. |
|
|
The operating unit is attached to the front of the bottom part with |
|
|
two hinges. This operating unit covers the area for the syringes. |
|
|
The complete drive assembly, consisting of lead screw and drive |
|
|
head with driving tube is located directly behind the syringe area |
|
|
in the bottom part of the housing. The housing bushing for the |
|
|
driving tube is located in the side of the housing. |
|
|
The syringe holder is mounted in the right side of the housing |
|
|
bottom part. |
|
|
The processor PCB with the permanently connected external |
|
|
connectors “P2” and “P3” is located at the bottom of the housing |
|
|
bottom part. |
|
Function |
There are two power options for the Perfusor® Space: |
|
— via the inserted battery module |
|
|
— via an external 12 V DC power supply (e.g. SpaceStation, |
|
|
SpaceControl, an external power supply or from an |
|
|
ambulance car) connected to connector “P2” |
|
|
The voltage supplied is converted to the internal voltages required |
|
|
through a voltage transforming and monitoring circuit on the |
|
|
processor PCB. |
|
|
An independent circuit in the battery module monitors the battery |
|
|
cells and controls their charge condition. |
|
|
The Perfusor® Space is connected to a SpaceControl by connector |
|
|
“P3”. |
|
|
The function processor controls all the functions of the Perfusor® |
|
|
Space. Data is stored in a non-volatile memory which also |
|
|
controls the external data transfer. |
|
|
The control microprocessor monitors all important responses of |
|
|
the function processor to incoming information. If a response |
|
|
does not correspond with that expected by the control |
|
|
microprocessor, an error message is generated and the device is |
|
|
switched to a safe stop state. |
|
|
The drive motor is monitored by a detector for speed and direction |
|
|
of rotation. The extended end position of the drive head is |
|
|
detected by a switch on the processor PCB. |
|
Perfusor® Space, 1.0 gb |
1 — 3 |

The pressure in the infusion system is measured through a strain gauge measuring in the drive head and monitored in the device electronics. The data from the strain gauge is continuously compared with the limit values which are calculated dependent on the selected syringe type and the pressure settings. When the limit values are exceeded an alarm is automatically triggered and the pressure in the infusion system is reduced. The maximum pressure is additionally limited by a second, independent system. This maximum pressure limitation is performed using the motor current control.
The syringe size detection is performed via the syringe holder. The syringe holder is connected to a potentiometer. The syringe size is determined from the resistance of the potentiometer.
The syringe is fixed with the syringe holder and the axial fastening device. The syringe piston is fastened with two claws in the drive head. When a syringe is inserted the syringe piston is held by the piston brake, until the piston has been caught by the claws.
Keyboard and display as well as the syringe area are illuminated.
|
1 — 4 |
Perfusor® Space, 1.0 gb |

|
System Overview |
1 |
|
Fig.: 1 — 3 Block diagram Perfusor® Space |
|
Perfusor® Space, 1.0 gb |
1 — 5 |

Unit Software |
Approved Software Versions |
|||||||||||||||
|
688A030032 |
||||||||||||||||
|
— |
Basic software |
|||||||||||||||
|
Position |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
||||||
|
Digit |
6 |
8 |
8 |
C |
0 |
3 |
0 |
0 |
0 |
1 |
688A030035 |
|||||
|
— |
Improved functions |
|||||||||||||||
|
688A030040 |
||||||||||||||||
|
Revision level |
||||||||||||||||
|
— |
Improved functions |
|||||||||||||||
|
Hardware |
||||||||||||||||
|
Software group |
— |
Languages French and Swedish added |
||||||||||||||
|
688B030002 |
||||||||||||||||
|
Device type: Perfusor® Space |
||||||||||||||||
|
Fig.: 1 — 4 |
— |
Improved functions |
||||||||||||||
|
688B030003 |
||||||||||||||||
|
— |
CAN bus functioning |
|||||||||||||||
|
688C030001 |
||||||||||||||||
|
— |
Dose calculation |
|||||||||||||||
|
— |
Changed CAN log |
|||||||||||||||
|
688D030001 |
||||||||||||||||
|
— Drug list data base |
||||||||||||||||
|
— |
Changed user language |
|||||||||||||||
|
688E030003 |
||||||||||||||||
|
— |
Improved functions |
|||||||||||||||
|
— |
Piggyback |
|||||||||||||||
|
— |
Soft limits |
|||||||||||||||
|
688F030006 |
||||||||||||||||
|
— |
PCA |
|||||||||||||||
|
— |
Changed claw configuration |
|||||||||||||||
|
— |
Optimized alarm handling |
|||||||||||||||
|
688G030002 |
||||||||||||||||
|
— |
Improved functions |
|
1 — 6 |
Perfusor® Space, 1.0 gb |

Service Program
Software Update of the Unit
The instructions for updating the software are supplied with the software itself.
CAUTION
If the device is disconnected while the software is being updated or the device or PC is switched off, a component of the software may be seriously damaged so that repairs are no longer possible. In such a case the software cannot be updated via the PC and the device must be returned to B. Braun.
Approved Version
Note
Please note that text and / or functions of the Service Program may change depending on the software version. The following screen illustrations are only examples and represent the state when the manual was printed.
—0.0.28
—1.0.0
—1.1.2
—1.1.3
—1.1.4
—1.2.1
—1.3.5
—1.5.0
—2.0.1
—3.1.0
—4.0.0
—5.1.0
Starting the Service Program
Note
Installation and further operation of the Service Program is described in its separate instructions for use.
|
Perfusor® Space, 1.0 gb |
1 — 7 |

Fig.: 1 — 5
Fig.: 1 — 6
Fig.: 1 — 7
1.Start the “HiBaSeD.exe” program (History, Barcode, Service, Drug list) on the PC. The Service Program is loaded and started and the initial window of the Service Program is displayed.
2.Read the notes carefully.
3.Mark the field “I accept all conditions” and then the field “Yes” to confirm that you have read the notes.
Note
Click the field “English” to switch the language of the notes over to English.
4.Enter the password and confirm it by clicking the field “Start”.
The Service Program checks the PC interfaces for connected devices of the Space system. Units that were found are displayed for a short moment on the screen.
|
1 — 8 |
Perfusor® Space, 1.0 gb |

The work window of the Service Program appears on the screen. All devices recognized are listed in the left column.
Fig.: 1 — 8
5. Activate the desired device from the list on the left in the work window with a double-click. The device data is then displayed below the device name.
|
Perfusor® Space, 1.0 gb |
1 — 9 |

If the unit software version is not compatible with the Service Program version, a window opens prompting the operator to change the Service Program version. This window displays a compatibility list of the Service Programand unit software versions.
If Service Programand unit software versions are compatible, all the Service Program functions are activated.
Fig.: 1 — 9
Fig.: 1 — 10
|
1 — 10 |
Perfusor® Space, 1.0 gb |

Fig.: 1 — 11
Fig.: 1 — 12
Service Program Version
1.Open the “HiBaSeD“ window via Help Info …. The current version of the Service Program is shown in this window.
2.Close the window by clicking “OK”.
Compatibility List
1.Open the “Unit — Compatibility” window via Help Compatibility. This window displays the compatibility of the HiBaSeD-version and the unit software version.
2.Close the window by clicking “OK”.
Quit the Service Program
1.Exit the Service Program via Application Quit.
2.Disconnect a power supply which might be connected from the unit.
3.Switch off the unit.
4.Remove the battery module.
5.The device can be restarted after appr. 10 seconds.
|
Perfusor® Space, 1.0 gb |
1 — 11 |

Technical Data
Options
Accessories
All technical data is indicated in the instructions for use.
The functions of the individual options are detailed in the instructions for use.
Perfusor® Space
Designation Part No.:
Power supply Euro . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0871 3110A Power supply UK. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0871 3111A Power supply USA / Japan . . . . . . . . . . . . . . . . . . . . . . 0871 3112A Power supply Australia. . . . . . . . . . . . . . . . . . . . . . . . . 0871 3113A Power supply South Africa. . . . . . . . . . . . . . . . . . . . . . 0871 3115A
|
Designation |
Part No.: |
|
Charger SP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
0871 3170 |
|
battery charging station |
|
|
Connection cable staff call SP. . . . . . . . . . . . . . . . . . . . |
0871 3232 |
|
Power supply cable 12 V . . . . . . . . . . . . . . . . . . . . . . . . |
0871 3231 |
|
for ambulance cars |
|
|
CombiLead SP 12 V . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
0871 3133 |
|
connection cable, pump — pump |
|
|
InterfaceLead SP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
0871 3234 |
|
interface cable RS232 |
|
|
InterfaceLead SP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
0871 3230 |
|
interface cable CAN SP |
|
|
SpaceClamp SP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
0871 3130 |
|
The SpaceClamp is a holder attached on beds for one |
|
|
or several Space system pumps. |
|
|
Short stand SP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
0871 3135 |
|
Space PCA kit (PCA button) . . . . . . . . . . . . . . . . . . . . . |
0871 3554 |
|
Syringe Anti Removal Cap PSP . . . . . . . . . . . . . . . . . . . |
0871 3556 |
|
1 — 12 |
Perfusor® Space, 1.3 gb |

Unit Diagnosis / Calibration 2
General
WARNING
WHILE TESTING THE UNIT AND TROUBLE SHOOTING THE
OPERATOR/SERVICE TECHNICIAN MUST WORK WITH VOLTAGES
UP TO 115 / 230 V AC. THESE VOLTAGES MAY CAUSE INJURIES
WHICH ARE DANGEROUS TO LIFE AND LIMB. THE NATIONAL AND
INTERNATIONAL SAFETY REGULATIONS ARE TO BE ADHERED TO.
Before each disassembly and assembly of a unit subsystem check the connectors, plug contacts and connections for corrosion and tight fit. These fault types are not described again in the following trouble shooting list.
The following equipment and gauges are necessary for testing the unit and/or performing troubleshooting:
— PC
— Service connector SP
— Service Program HiBaSeD — Interface cable
— Syringe 2 ml / 3 ml — Syringe 10 ml
— Syringe 30 ml
— Diameter gauge 32.0 mm — Diameter gauge 23.4 mm — Diameter gauge 15.7 mm — Diameter gauge 9.0 mm — Length gauge PSP
— Syringe gauge “#Lehre OPS 50“with push-button plate and motor power test adapter for Perfusor® Space
There are pictures of the gauges in Chapter “Special Tools“ ( pg. 8 — 3).
CAUTION
Take special care when carrying out measurements on an open and switched-on unit. Short circuits and wrong measuring methods can cause serious damage to or destroy the subsystems of the device.
|
Perfusor® Space, 1.1 gb |
2 — 1 |

|
2 |
Unit Diagnosis / Calibration |
The unit check, calibration and trouble shooting are subdivided into numbered working steps (Unit Test Step UTS, Calibration Step CS, Trouble Shooting TS) and are based on each other.
Beginning with UTS 1 the operation described here has to be executed. The consequences of the steps performed are listed in the “Function“ column. If the result corresponds to the consequence, the working step must be carried out to which reference is made in the column “If yes”. If the result does not correspond with the function described, the working step in column “If no” is to be executed.
One example is given in Fig.: 2 — 1.
|
UTS |
Activity |
Function |
If yes |
If no |
|
1 |
UTS 2 |
|||
|
2 |
UTS 3 |
TS 1 |
||
|
3 |
UTS 4 |
|||
|
4 |
UTS 5 |
TS 4 |
||
|
5 |
||||
|
Model table 1 |
||||
|
TS |
Activity |
Function |
||
|
1 |
UTS 3 |
TS 2 |
||
|
2 |
TS 3 |
TS 4 |
||
|
3 |
UTS 3 |
|||
|
4 |
UTS 4 |
TS 4 |
||
|
5 |
UTS 4 |
|||
|
Model table 2 |
||||
|
Fig.: 2 — 1 Model tables |
Steps for which additional information is required are described after the table in detail.
|
2 — 2 |
Perfusor® Space, 1.0 gb |

|
Unit Diagnosis / Calibration |
2 |
Alarms and Error Codes
The alarms of the Perfusor® Space are classified in 5 categories. These categories are listed hereafter according to their importance.
—Alarm advice
In case of unacceptable inputs corresponding messages are displayed (e.g. “Caution! Rate out of range“, “The parameter cannot be changed“) and a beep sounds.
—Pre-alarm
Pre-alarms are triggered several minutes (depending on the service settings) before the operating alarms.
—Reminder alarm
A reminder alarm is triggered if the device is not operated for two minutes when input or operation was not finished.
—Operating alarm
In case of an operating alarm the infusion is stopped. An audible signal is released, the red LED flashes and a staff call is triggered. The message “Alarm” and the cause of the alarm appear on the display.
—Device alarm
The most important alarms and error codes as well as their meaning and possible fault clearance are specified in the following lists.
Note
The device should be checked after every repair or service (see “Device Check“ pg. 2 — 9).
|
Perfusor® Space, 1.0 gb |
2 — 3 |

|
2 |
Unit Diagnosis / Calibration |
|
Alarms |
|||
|
Alarm |
Possible Cause |
Fault Clearance |
|
|
1 |
Battery nearly discharged (type: pre- |
The device was not connected to the |
Operate the device with battery until the |
|
alarm) |
mains long enough |
message “Battery discharged“ is |
|
|
displayed and the unit is switched off. |
|||
|
Then connect the unit to the mains for at |
|||
|
least 6 hours. |
|||
|
Battery module defective or too old |
Replace battery module |
||
|
2 |
Battery discharged (type: operating |
The device was not connected to the |
Connect the unit to the mains for at least |
|
alarm) |
mains long enough |
6 hours |
|
|
Battery module defective |
Replace battery module |
||
|
3 |
Battery cover open (type: operating |
The battery compartment cover is |
Insert the battery compartment cover |
|
alarm) |
not correctly closed |
correctly |
|
|
The magnet in the battery |
Exchange the battery compartment cover |
||
|
compartment cover is missing |
|||
|
The battery compartment cover is |
Replace battery module |
||
|
not recognized by the battery |
|||
|
module |
|||
|
4 |
Drive blocked (type: operating alarm) |
The drive was manually blocked |
Eliminate blockage |
|
Driving force too low |
Connect the unit to the mains for at least |
||
|
6 hours and charge battery |
|||
|
Re-calibrate the device |
|||
|
The drive is physically damaged |
Replace drive. |
||
|
5 |
Malfunction of claws (type: operating |
The syringe piston was not |
Select or insert correct syringe type |
|
alarm) |
recognized |
||
|
Loosen the syringe via the emergency |
|||
|
release button in the drive head and |
|||
|
insert again |
|||
|
Re-calibrate the device |
|||
|
The claws or the claw drive are/is |
Replace drive head |
||
|
damaged |
|||
|
6 |
Push-button has no contact (type: |
Negative pressure in the syringe |
See instructions for use |
|
operating alarm) |
system |
||
|
Syringe was removed without |
See instructions for use |
||
|
opening the syringe holder |
|||
|
Push-button sensor defective |
Replace drive head |
||
|
7 |
Device alarm (type: device alarm) |
A serious internal fault was detected in |
Switch device off and on |
|
the system |
|||
|
Carry out a device check (see “Device |
|||
|
Check“ pg. 2 — 9) |
|||
|
Table 2 — 1 Alarms |
|
2 — 4 |
Perfusor® Space, 1.0 gb |

|
Unit Diagnosis / Calibration |
2 |
|
Device Alarms of the Function Processor |
||||
|
Error Code |
Definition |
Possible Cause |
Fault Clearance |
|
|
1 |
1001 … 1013 |
Internal Error |
||
|
2 |
1014 |
Loudspeaker not off |
Loudspeaker connector |
Check the loudspeaker connector |
|
Loudspeaker |
Check the loudspeaker |
|||
|
3 |
1015 |
Loudspeaker lost |
Loudspeaker connector |
Check the loudspeaker connector |
|
Loudspeaker |
Check the loudspeaker |
|||
|
4 |
1016 |
Loudspeaker shorted |
Loudspeaker connector |
Check the loudspeaker connector |
|
Loudspeaker |
Check the loudspeaker |
|||
|
5 |
1017 |
KuP switchoff path defect |
Switch off path |
|
|
(K_SM_CLK) |
||||
|
6 |
1018 |
ADC pressure out of range |
Pressure measurement in drive |
Carry out calibration |
|
head |
||||
|
7 |
1019 |
Internal Error |
||
|
8 |
1020 |
FUP Flash Memory Error Software |
Software |
Update unit software |
|
9 |
1021 |
FUP different version KuP to FuP |
Software |
Update unit software |
|
10 |
1022 |
FUP pressure zero test fail |
Pressure measurement in drive |
Carry out calibration |
|
head |
||||
|
11 |
1023 |
FUP pressure offset test fail |
Pressure measurement in drive |
Carry out calibration |
|
head |
||||
|
12 |
1024 |
FUP EA key closed too long 20sec |
Keyboard defective |
Carry out device check |
|
13 |
1025 |
Internal Error |
||
Table 2 — 2 Device alarms of the function processor
|
Perfusor® Space, 1.0 gb |
2 — 5 |

|
2 |
Unit Diagnosis / Calibration |
|
Device Alarms of the Control Microprocessor |
||||
|
Error Code |
Definition |
Possible Cause |
Fault Clearance |
|
|
1 |
1100 |
Timebase too fast |
Quartz of the processor PCB |
Exchange processor PCB |
|
2 |
1101 |
Timebase too slow |
Quartz of the processor PCB |
Exchange processor PCB |
|
3 |
1102 |
Timebase fail |
Quartz of the processor PCB |
Exchange processor PCB |
|
4 |
1103 |
Keyboard High |
Keyboard defective |
Carry out device check |
|
5 |
1104 |
EA_KEY defect 25sec |
Keyboard defective |
Carry out device check |
|
6 |
1105 |
No keydecode |
Keyboard defective |
Carry out device check |
|
7 |
1106 |
ROM Romtest defect Software |
Software |
Update unit software |
|
8 |
1107 |
ROM Program defect |
Software |
Update unit software |
|
9 |
1108 |
CM State without set |
||
|
K_V_KM_ON |
||||
|
10 |
1109 |
MPU_Test failed |
Software |
Update unit software |
|
11 |
1110 |
RAM_Test failed |
Software |
Update unit software |
|
12 |
1111 |
active reset |
Voltage supply during operation |
|
|
interrupted |
||||
|
13 |
1112 … 1114 |
Internal Error |
||
|
14 |
1115 |
Drive too fast |
Motor drive |
Exchange processor PCB |
|
Recognition of direction of |
||||
|
rotation |
||||
|
15 |
1116 |
Drive too slow |
Motor drive |
Exchange processor PCB |
|
Recognition of direction of |
||||
|
rotation |
||||
|
16 |
1117 … 1118 |
Internal Error |
||
|
17 |
1119 |
lcd backlight on defect |
LC display defective |
Exchange operating unit |
|
18 |
1120 |
lcd backlight off defect |
LC display defective |
Exchange operating unit |
|
19 |
1121 |
red led on defect |
LC display defective |
Exchange operating unit |
|
20 |
1122 |
red led off defect |
LC display defective |
Exchange operating unit |
|
21 |
1123 |
key pressed too long (without EA- |
Keyboard defective |
Carry out device check |
|
Key) 60sec |
||||
|
22 |
1124 … 1127 |
Internal Error |
||
|
23 |
1128 |
Drive motion rightless forward |
Motor drive |
Exchange processor PCB |
|
Recognition of direction of |
||||
|
rotation |
||||
|
24 |
1129 |
Drive motion rightless backward |
Motor drive |
Exchange processor PCB |
|
Recognition of direction of |
||||
|
rotation |
||||
|
25 |
1130 … 1200 |
Internal Error |
||
Table 2 — 3 Device alarms of the control microprocessor (Part 1 of 2)
|
2 — 6 |
Perfusor® Space, 1.0 gb |

|
Unit Diagnosis / Calibration |
2 |
|
Error Code |
Definition |
Possible Cause |
Fault Clearance |
|
|
26 |
1201 |
different version FuP to KuP |
Software |
Update unit software |
|
Software |
||||
|
27 |
1202 |
E_ERROR_STEPMOTOR_1 Phase |
Drive motor, lead screw |
Exchange processor PCB |
|
not ok |
||||
|
28 |
1203 |
E_ERROR_STEPMOTOR_2 Current |
Motor drive |
Carry out calibration |
|
value not 0x55 |
Recognition of direction of |
|||
|
rotation |
||||
|
29 |
1204 |
E_ERROR_STEPMOTOR_3 |
Motor drive |
Carry out calibration |
|
K_SM_CLK defect |
Recognition of direction of |
|||
|
rotation |
||||
|
30 |
1205 |
E_ERROR_STEPMOTOR_4 Phase |
Motor drive |
Carry out calibration |
|
not ok |
Recognition of direction of |
|||
|
rotation |
||||
|
31 |
1206 |
E_ERROR_STEPMOTOR_5 |
Motor drive |
Carry out calibration |
|
Current value not 0 |
Recognition of direction of |
|||
|
rotation |
||||
|
32 |
1207 |
E_ERROR_STEPMOTOR_6 Current |
Motor drive |
Carry out calibration |
|
value not 0x55 |
Recognition of direction of |
|||
|
rotation |
||||
|
33 |
1208 |
E_ERROR_STEPMOTOR_7 Current |
Motor drive |
Carry out calibration |
|
value not 0xAA |
Recognition of direction of |
|||
|
rotation |
||||
|
34 |
1209 |
E_ERROR_STEPMOTOR_8 Phases |
Motor drive |
Carry out calibration |
|
not 0 |
Recognition of direction of |
|||
|
rotation |
||||
|
35 |
1210 |
E_ERROR_DCMOTOR_1 |
Piston brake drive motor def. |
|
|
Claw drive in drive head defective |
||||
|
36 |
1211 |
E_ERROR_DCMOTOR_2 |
||
|
Piston brake light barrier def. |
||||
|
37 |
1212 |
E_ERROR_DCMOTOR_3 |
||
|
38 |
1213 |
E_ERROR_DCMOTOR_4 |
||
|
39 |
1214 |
E_ERROR_DCMOTOR_5 |
||
|
40 |
1215 |
no V_MOT |
Voltage transformer defective |
Exchange processor PCB |
|
41 |
1216 |
overvoltage test fail |
||
|
42 |
1217 |
no V_MOT |
||
|
43 |
1218 |
undervoltage test fail |
||
|
44 |
1220 |
syringeholder defect |
Syringe holder or potentiometer |
Replace syringe holder |
|
def. |
Exchange processor PCB |
|||
|
45 |
1221 |
syringe change timeout |
||
|
46 |
1237 … 1238 |
Internal Error |
||
|
47 |
1239 |
plunger plate sensor defect |
Pressure measurement in drive |
Replace drive head |
|
head |
||||
|
48 |
1240 … 1254 |
Internal Error |
||
Table 2 — 3 Device alarms of the control microprocessor (Part 2 of 2)
|
Perfusor® Space, 1.0 gb |
2 — 7 |

|
2 |
Unit Diagnosis / Calibration |
The Most Important Error Modes |
The following list specifies the most important error modes and |
||||
|
their clearance. |
|||||
|
Note |
|||||
|
The device must be checked after every repair or service (see |
|||||
|
“Device Check“ pg. 2 — 9). |
|||||
|
Error |
Possible Cause |
Fault Clearance |
|||
|
1 |
The battery module discharges too fast |
The device was not used for a longer time. |
Discharge and charge battery module |
||
|
The battery module was not discharged |
several times |
||||
|
and charged at regular intervals. |
Replace battery module |
||||
|
Table 2 — 4 |
|
2 — 8 |
Perfusor® Space, 1.0 gb |

|
Unit Diagnosis / Calibration |
2 |
Device Check
|
UTS |
Activity |
Function |
If yes |
If no |
|
|
1 |
The device is inserted in a SpaceStation or |
UTS 2 |
UTS 3 |
||
|
connected to a SpaceControl. |
|||||
|
2 |
Remove the device. |
UTS 4 |
|||
|
3 |
Loosen all connections from the device. |
UTS 4 |
|||
|
4 |
Remove syringe and close syringe holder. |
UTS 5 |
|||
|
5 |
Plug service connector SP on connector “P2”. |
UTS 6 |
|||
|
6 |
Connect power supply to the device via service |
All LEDs light up for a short moment. |
UTS 7 |
TS 1 |
|
|
connector SP. |
|||||
|
7 |
The battery charge state and the mains connection |
UTS 8 |
TS 5 |
||
|
are displayed at the top left of the LC display |
|||||
|
(without lighting). |
|||||
|
8 |
Switch on unit. |
All LEDs light up (from left: yellow, green, blue). |
UTS 9 |
TS 5 |
|
|
9 |
A short deep and then a short high beep sound. |
UTS 10 |
TS 7 |
||
|
10 |
The colour of the middle LED changes from green to |
UTS 11 |
TS 8 |
||
|
red, then the LED goes out. The yellow and the blue |
|||||
|
LED remain on for a short moment. |
|||||
|
11 |
The message “Self-test active” and the current |
UTS 12 |
TS 8 |
||
|
software version are displayed. |
|||||
|
12 |
Keyboard, LC display as well as the syringe area are |
UTS 13 |
TS 9 |
||
|
illuminated. |
|||||
|
13 |
The drive head moves to the extended end position. |
UTS 14 |
TS 11 |
||
|
14 |
The claws in the syringe head close and open. |
UTS 15 |
TS 14 |
||
|
15 |
The message “Drive moves back / Syringe change” |
UTS 16 |
TS 16 |
||
|
appears on the display. |
|||||
|
16 |
“Open syringe holder and insert syringe or press “C“ |
UTS 17 |
TS 16 |
||
|
to input parameters“ is displayed. |
|||||
|
17 |
Open syringe holder. |
“Syringe change / Please insert syringe …” is |
UTS 18 |
TS 17 |
|
|
displayed. |
|||||
|
18 |
Press the “>” key. |
The service information: |
UTS 19 |
TS 19 |
|
|
— Brake: not started or active |
|||||
|
— drivetest ok |
|||||
|
— Size: 35.4 KuP 35.4 FuP |
|||||
|
is displayed on the LC display. |
|||||
|
19 |
Insert syringe 30 ml. |
The syringe piston is fastened with the syringe |
UTS 20 |
TS 21 |
|
|
holder blade. |
|||||
|
20 |
On the LC display “Brake: stopped by current” |
UTS 21 |
TS 21 |
||
|
appears in the line. |
|||||
|
Table 2 — 5 Device check |
(Part 1 of 5) |
|
Perfusor® Space, 1.1 gb |
2 — 9 |

|
2 |
Unit Diagnosis / Calibration |
|
UTS |
Activity |
Function |
If yes |
If no |
|
21 |
Insert 2 ml / 3 ml syringe. |
On the LC display “Brake: stopped by holder” |
UTS 22 |
TS 21 |
|
appears in the line. |
||||
|
22 |
Open syringe holder and remove syringe. |
On the LC display “Brake: stopped by light barrier” |
UTS 23 |
TS 21 |
|
appears in the line. |
||||
|
23 |
3.54 is shown for FuP on the LC display. The value |
UTS 24 |
CS 1 |
|
|
displayed for FuP may have a maximum tolerance of |
||||
|
± 0.04. |
||||
|
24 |
Close syringe holder. |
The value for FuP changes to 7.0 ± 0.4. |
UTS 25 |
CS 1 |
|
25 |
Insert diameter gauge 9.0 mm. |
The value for FuP changes to 9.0 ± 0.4. |
UTS 26 |
CS 1 |
|
26 |
Insert diameter gauge 15.7mm. |
The value for FuP changes to 15.7 ± 0.4. |
UTS 27 |
CS 1 |
|
27 |
Insert diameter gauge 23.4 mm. |
The value for FuP changes to 23.4 ± 0.4. |
UTS 28 |
CS 1 |
|
28 |
Insert diameter gauge 32.0 mm. |
The value for FuP changes to 32.0 ± 0.4. |
UTS 29 |
CS 1 |
|
29 |
The sum of the tolerances of UTS 23 to UTS 28 must |
UTS 30 |
CS 1 |
|
|
not exceed 1.0. |
||||
|
30 |
Insert 2 ml / 3 ml syringe. |
UTS 31 |
||
|
31 |
Press the “>” key. |
The syringe selection is displayed. |
UTS 32 |
|
|
32 |
Select a syringe. |
The drive head moves to the syringe piston, the |
UTS 33 |
TS 26 |
|
claws in the drive head close and the message |
||||
|
“Syringe is caught / Please wait” is displayed. |
||||
|
33 |
Test all buttons on the operating unit during a |
When the buttons are pressed the desired reaction |
UTS 34 |
TS 29 |
|
functional check (carry out infusion). |
is carried out. |
|||
|
34 |
Open syringe holder while the infusion is |
The red LED on the operating unit flashes and the |
UTS 35 |
TS 31 |
|
administered. |
red LED of the service connector SP lights up. The |
|||
|
message “Alarm / Syringe holder” is displayed. |
||||
|
35 |
Close syringe holder and continue infusion. |
UTS 36 |
||
|
36 |
Stop infusion. |
UTS 37 |
||
|
37 |
Open syringe holder. |
“Syringe change / Initiate change? Yes / No” is |
UTS 38 |
|
|
displayed. |
||||
|
38 |
Confirm with “Yes”. |
The claws in the drive head open and the drive head |
UTS 39 |
|
|
moves to the extended end position. |
||||
|
39 |
Remove syringe. |
UTS 40 |
||
Table 2 — 5 Device check (Part 2 of 5)
|
2 — 10 |
Perfusor® Space, 1.1 gb |

|
Unit Diagnosis / Calibration |
2 |
|
UTS |
Activity |
Function |
If yes |
If no |
||
|
40 |
Insert syringe gauge for the strain gauge |
UTS 41 |
||||
|
measurement, close syringe holder and select |
||||||
|
syringe type „#Lehre OPS50“. The syringe gauge |
||||||
|
must not be tipped. Therefore fix the syringe gauge |
||||||
|
so far into the syringe recess by hand that the |
||||||
|
piston brake moves back and the claws surrounds |
||||||
|
the pressure element. |
||||||
|
WARNING |
||||||
|
DURING THE STRAIN GAUGE MEASUREMENT WITH |
||||||
|
SYRINGE GAUGE THE SYRINGE HOLDER MUST NOT |
||||||
|
BE OPENED. THE SYRINGE GAUGE IS UNDER VERY |
||||||
|
HIGH PRESSURE AND MAY CAUSE INJURIES IF THE |
||||||
|
PRESSURE IS RELIEVED SUDDENLY. |
||||||
|
41 |
Input a delivery rate of 200 ml/h, select pressure |
When the maximum pressure of this pressure stage |
UTS 42 |
CS 1 |
||
|
stage 1 and start infusion. |
is reached, the delivery is stopped, the red LED on |
|||||
|
the operating unit flashes and the message “Alarm |
||||||
|
/ Pressure too high” is displayed. |
||||||
|
The value read on the syringe gauge (in N) must |
||||||
|
match the value indicated for the strain gauge |
||||||
|
measurement of this pressure stage in the TSC. |
||||||
|
42 |
Confirm alarm. |
UTS 43 |
||||
|
43 |
Select pressure stage 3 and start infusion. |
When the maximum pressure of this pressure stage |
UTS 44 |
CS 1 |
||
|
is reached, the delivery is stopped, the red LED on |
||||||
|
the operating unit flashes and the message “Alarm |
||||||
|
/ Pressure too high” is displayed. |
||||||
|
The value read on the syringe gauge (in N) must |
||||||
|
match the value indicated for the strain gauge |
||||||
|
measurement of this pressure stage in the TSC. |
||||||
|
44 |
Confirm alarm. |
UTS 45 |
||||
|
45 |
Select pressure stage 8 and start infusion. |
When the maximum pressure of this pressure stage |
UTS 46 |
CS 1 |
||
|
is reached, the delivery is stopped, the red LED on |
||||||
|
the operating unit flashes and the message “Alarm |
||||||
|
/ Pressure too high” is displayed. |
||||||
|
The value read on the syringe gauge (in N) must |
||||||
|
match the value indicated for the strain gauge |
||||||
|
measurement of this pressure stage in the TSC. |
||||||
|
46 |
Confirm alarm and pull syringe holder briefly. |
UTS 47 |
||||
Table 2 — 5 Device check (Part 3 of 5)
|
Perfusor® Space, 1.3 gb |
2 — 11 |

|
2 |
Unit Diagnosis / Calibration |
|
UTS |
Activity |
Function |
If yes |
If no |
||
|
47 |
Confirm syringe change, release syringe gauge and |
UTS 48 |
||||
|
remove gauge. |
||||||
|
WARNING |
||||||
|
WHILE CHECKING THE MOTOR POWER LIMITATION |
||||||
|
WITH THE SYRINGE GAUGE THE SYRINGE HOLDER |
||||||
|
MUST NOT BE OPENED. THE SYRINGE GAUGE IS |
||||||
|
UNDER VERY HIGH PRESSURE AND MAY CAUSE |
||||||
|
INJURIES IF THE PRESSURE IS RELIEVED SUDDENLY. |
||||||
|
48 |
Insert the motor power test adapter in the drive |
UTS 49 |
||||
|
head to check the motor power limitation. |
||||||
|
49 |
Dismount the push-button plate from the syringe |
UTS 50 |
||||
|
gauge and insert syringe gauge. |
||||||
|
50 |
Select syringe type “#Lehre OPS 50“. The threaded |
UTS 51 |
||||
|
end of the syringe gauge must be introduced in the |
||||||
|
opening of the motor power test adapter. To do this, |
||||||
|
hold on to the syringe gauge, if necessary by hand, |
||||||
|
in the syringe area. |
||||||
|
51 |
Select pressure stage 1 and start infusion. |
When the maximum pressure of this pressure stage |
UTS 52 |
CS 1 |
||
|
is reached, the delivery is stopped, the red LED on |
||||||
|
the operating unit flashes and the message “Alarm |
||||||
|
/ Drive blocked” is displayed. |
||||||
|
The value read on the syringe gauge (in N) must |
||||||
|
match the value indicated for the motor power |
||||||
|
limitation in the TSC. |
||||||
|
52 |
Confirm alarm. |
UTS 53 |
||||
|
53 |
Select pressure stage 3 and start infusion. |
When the maximum pressure of this pressure stage |
UTS 54 |
CS 1 |
||
|
is reached, the delivery is stopped, the red LED on |
||||||
|
the operating unit flashes and the message “Alarm |
||||||
|
/ Drive blocked” is displayed. |
||||||
|
The value read on the syringe gauge (in N) must |
||||||
|
match the value indicated for the motor power |
||||||
|
limitation in the TSC. |
||||||
|
54 |
Confirm alarm. |
UTS 55 |
||||
Table 2 — 5 Device check (Part 4 of 5)
|
2 — 12 |
Perfusor® Space, 1.0 gb |

|
Unit Diagnosis / Calibration |
2 |
|
UTS |
Activity |
Function |
If yes |
If no |
||
|
55 |
Select pressure stage 6 and start infusion. |
When the maximum pressure of this pressure stage |
UTS 56 |
CS 1 |
||
|
is reached, the delivery is stopped, the red LED on |
||||||
|
the operating unit flashes and the message “Alarm |
||||||
|
/ Drive blocked” is displayed. |
||||||
|
The value read on the syringe gauge (in N) must |
||||||
|
match the value indicated for the motor power |
||||||
|
limitation in the TSC. |
||||||
|
56 |
Confirm alarm and pull syringe holder briefly. |
UTS 57 |
||||
|
57 |
Confirm syringe change, release syringe gauge and |
UTS 58 |
||||
|
remove gauge. |
||||||
|
WARNING |
||||||
|
WHILE CHECKING THE MOTOR POWER LIMITATION |
||||||
|
WITH THE SYRINGE GAUGE THE SYRINGE HOLDER |
||||||
|
MUST NOT BE OPENED. THE SYRINGE GAUGE IS |
||||||
|
UNDER VERY HIGH PRESSURE AND MAY CAUSE |
||||||
|
INJURIES IF THE PRESSURE IS RELIEVED SUDDENLY. |
||||||
|
58 |
Insert syringe type 50/60 ml and lock PCA-lock with |
The syringe holder cannot be opened. |
UTS 59 |
TS 32 |
||
|
PCA-key. |
||||||
|
59 |
Open PCA-lock and remove syringe. |
UTS 60 |
||||
|
60 |
Insert syringe type 10 ml and lock PCA-lock with |
The syringe holder cannot be opened. |
UTS 61 |
TS 32 |
||
|
PCA-key. |
||||||
|
61 |
Open PCA-lock and remove syringe. |
UTS 62 |
||||
|
62 |
Switch device off. |
The message “Pump is switched off in 3 .. 2 .. 1 sec” |
UTS 63 |
|||
|
is displayed. |
||||||
|
63 |
“Drive is parked …/ Please wait …“ is displayed. The |
UTS 64 |
||||
|
drive head moves to the retracted park position. |
||||||
|
64 |
The device switches off. |
UTS 65 |
TS 35 |
|||
|
65 |
Pull off the power supply. |
The blue LED lights up for a short moment. |
UTS 66 |
TS 35 |
||
|
66 |
Switch on unit. |
UTS 67 |
||||
|
67 |
Open the battery compartment cover when the |
An alarm signal sounds, the red LED flashes and |
UTS 68 |
TS 36 |
||
|
drive head has moved to the extended end position. |
“Alarm / Battery cover open / Confirm with “OK” is |
|||||
|
displayed. |
||||||
|
68 |
Remove battery. |
A permanent alarm is triggered. |
UTS 69 |
TS 38 |
||
|
69 |
Insert battery, close battery compartment cover and |
The message “Devicealarm / 1111” is displayed. |
UTS 70 |
|||
|
switch on the device. |
||||||
|
70 |
Switch the device off, remove service connector SP |
This step |
terminates |
|||
|
and dismount test structure. |
the device check. |
|||||
Table 2 — 5 Device check (Part 5 of 5)
|
Perfusor® Space, 1.3 gb |
2 — 13 |

|
2 |
Unit Diagnosis / Calibration |
Calibration
|
CS |
Activity |
Function |
If yes |
If no |
|
1 |
Connect unit to PC with interface cable. |
CS 2 |
||
|
2 |
Start Service Program on the PC (see “Starting the |
The desired device is found by the Service Program |
CS 3 |
|
|
Service Program“ pg. 1 — 7). |
and then displayed. |
|||
|
3 |
Start calibrating the unit (see “Starting Calibration“ |
CS 4 |
||
|
pg. 2 — 14). |
||||
|
4 |
Carry out calibration of the claws (see “Claw |
Calibration of the claws was terminated |
CS 5 |
|
|
Calibration“ pg. 2 — 23). |
successfully. |
|||
|
5 |
Carry out calibration of the syringe holder (see |
Calibration of the syringe holder was terminated |
CS 6 |
|
|
“Syringe Holder Calibration“ pg. 2 — 23). |
successfully. |
|||
|
6 |
Carry out pressure calibration (see “Pressure |
Pressure calibration was terminated successfully. |
CS 7 |
|
|
Calibration“ pg. 2 — 26). |
||||
|
7 |
Close the Service Program (see “Quit the Service |
UTS 23 |
||
|
Program“ pg. 1 — 11). |
||||
Table 2 — 6 Calibration
Procedural Instructions for Calibration
Starting Calibration
Note
Calibration must be carried out with power supply connected, since the calibration can be interrupted suddenly if the unit is battery-operated and the battery gets discharged so that the device is switched off.
Note
Please note that text and / or functions of the Service Program may change depending on the software version. The following screen illustrations are only examples and represent the state when the manual was printed.
|
2 — 14 |
Perfusor® Space, 1.1 gb |

|
Unit Diagnosis / Calibration |
2 |
1. Start the Service Program (see “Starting the Service Program“pg. 1 — 7).
2. Select the unit to be calibrated in the left column of the window with a double mouse-click. The blue and the yellow LED blinks in opposite with the red LED.
3. Select the register tab “Calibration”.
Fig.: 2 — 2
4. Press the “New device” button in the frame “Calibration procedure”. The window “Worker ID” is opened.
Note
If you do not have an allocated worker id, enter “0001”.
|
Perfusor® Space, 1.2 gb |
2 — 15 |

|
2 |
Unit Diagnosis / Calibration |
Fig.: 2 — 3
Fig.: 2 — 4
Fig.: 2 — 5
5.Input your user number in the window “Worker ID” as well as the six-digit serial number of the device, if necessary.
6.Confirm the input with “OK”.
Note
If HiBaSeD could not clearly read the device serial number, the number must be entered according to the rating plate.
If the unit is not yet switched on, the window “Device on/off” opens and the user is asked to select the desired language.
7.Select the desired language. The respective operating steps are explained in detail in the instructions for use.
After the language was confirmed the unit switches on and the window “Device on/off” closes.
Note
If calibration is interrupted, data is written back to the device and marked as invalid if this is still possible. When the Service Program is started again the data is marked as faulty and highlighted red when the “Modify” button is selected in the “Calibration procedure” frame.
|
2 — 16 |
Perfusor® Space, 1.2 gb |
 Loading…
Loading…
You can only view or download manuals with
Sign Up and get 5 for free
Upload your files to the site. You get 1 for each file you add
Get 1 for every time someone downloads your manual
Buy as many as you need
 Loading…
Loading…
You can only view or download manuals with
Sign Up and get 5 for free
Upload your files to the site. You get 1 for each file you add
Get 1 for every time someone downloads your manual
Buy as many as you need
Автоматизированная шприцевая инфузионная система Perfusor Space B Braun состоит из переносного электронного шприцевого насоса и принадлежностей для него. Система предназначена для проведения терапии у взрослых, детей и новорожденных, проведения периодического или непрерывного парентерального или энтерального введения растворов через клинически обусловленные доступы. Эти доступы включают внутривенный, внутриартериальный, подкожный, эпидуральный и энтеральный.

Система применяется для введения медикаментов, предназначенных для инфузионной терапии, включая анестетики, седативные, анальгетики, катехоламины, антикоагулянты и пр.; кровь и компоненты крови; полное парентеральное питание (TPN); жиры; энтеральные смеси, но не ограничивается только ими. Автоматизированная шприцевая инфузионная система Перфузор Спэйс Б. Браун предназначена для применения подготовленным медицинским персоналом в стационарных и амбулаторных лечебных учреждениях, на дому и в санитарном транспорте. Решение о применении данной системы должно приниматься квалифицированным медицинским персоналом на основании ее свойств и технических характеристик.
Производитель Perfusor Space B Braun
Производитель Перфузор Спэйс Б. Браун – B. Braun Melsungen AG, Германия. Представительство в России Б. Браун Медикал ООО, Санкт-Петербург Россия, тел. (812) 320-40-04.
Perfusor Space: Обзор
Ниже представлено описание кнопок и деталей автоматизированной шприцевой инфузионной системы Perfusor Space B Braun.
Вид спереди
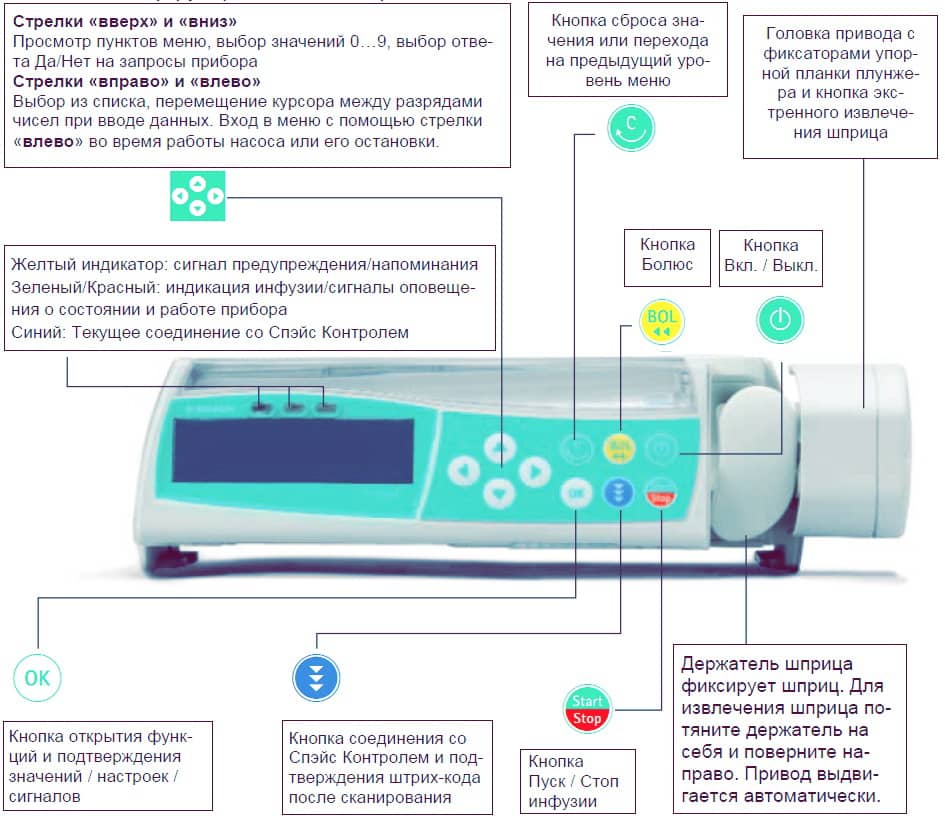
Вид сзади

Фиксация шприца
Вытяните и поверните вправо держатель шприца для открытия зеленого осевого фиксатора (см. стрелку). Перед закрытием держателя установите упорную планку цилиндра шприца вертикально в паз слева от осевого фиксатора. Убедитесь в правильном положении шприца. Не касайтесь фиксатора штока при закрытии держателя шприца.
Фиксация универсального зажима
Совместите пазы в верхней части насоса с направляющими универсального зажима и двигайте рамку универсального зажима вперед до щелчка фиксатора. Для отсоединения, нажмите на кнопку извлечения на рамке, опустите транспортную ручку рамки универсального зажима и сдвиньте рамку назад.
Соединение Perfusor Space
До трех насосов (Инфузомат Спэйс B braun или Перфузор Спэйс B braun) с модулем Спэйс Контроль могут стыковаться в единый блок. Остерегайтесь внешнего механического воздействия. Для соединения приборов совместите пазы нижнего насоса с направляющими верхнего и двигайте нижний насос назад до щелчка фиксатора и сопоставления зеленых кнопок друг под другом. Для отсоединения, нажмите на зеленый боковой фиксатор на верхнем насосе и выдвиньте нижний насос.
Фиксация на стойке

Установите зажим на вертикальной стойке и надежно закрутите винтовой фиксатор. Для снятия, открутите фиксатор. Фиксация на горизонтальной стойке: нажмите на рычаг и вращайте рамку в нужную сторону до фиксации в новом положении со щелчком. Для смены положения снова отожмите рычаг.
Управление Perfusor Space
- Первичный инструктаж по работе с Перфузором Спэйс проводится представителем Б. Браун или уполномоченным лицом. После обновления программного обеспечения Пользователь должен быть проинформирован об изменениях в работе прибора и принадлежностей, описанных в Руководстве по применению.
- Убедитесь, что прибор надежно установлен и закреплен. Не устанавливайте насос над пациентом.
- Перед применением осмотрите насос для исключения повреждений, некомплекта или загрязнений, проверьте аудиовизуальные сигналы самотестирования.
- Подключение к пациенту разрешается только после того, как шприц правильно установлен в насос, зафиксирован захватами привода и линия заполнена раствором. Перекрывайте соединение во время смены шприца для предупреждения неправильного дозирования лекарства.
- Выберите шприц/катетер сообразно предстоящему применению.
- Установите инфузионную линию, избегая перегибов.
- Рекомендуется менять расходные материалы каждые 24 часа (или в соответствие с государственными гигиеническими нормативами).
- Установка в медицинских помещениях должна соответствовать существующим требованиям (напр. VDE 0100, VDE 0107 или постановлениям IEC). Соблюдайте установленные требования и нормативы по безопасности.
- Для предупреждения риска взрыва, не используйте насос в зоне применения огнеопасных анестетиков.
Безопасность пациента
- Сравните введенные параметры с данными экрана. Начинайте инфузию только при полном соответствии параметров.
- При использовании системы вызова персонала, рекомендуется ее проверка после подключения инфузионных насосов.
- Оберегайте прибор и кабель питания от воздействия влаги.
- Не держите насос за привод при переноске.
- В случае падения насоса или внешнего силового воздействия на него, прибор должен быть проверен сервисной службой.
- Данные, отображаемые на экране должны всегда проверяться персоналом до принятия следующего клинического решения.
- Применение насоса как мобильного устройства (при домашнем уходе, внутри- и внебольничной транспортировке пациента): Убедитесь, что прибор надежно установлен и закреплен. Смена положения и сильное сотрясение могут привести к незначительным изменениям точности инфузии.
- При проведении инфузионной терапии в критических ситуациях, обязателен дополнительный мониторинг пациента.
- Избегайте внешнего воздействия на привод во время инфузии.
- При введении высокоактивных лекарств, подготовьте второй насос с тем же лекарством.
- Независимо от заданных мягких ограничений, вводимые значения должны соответствовать клиническому состоянию данного пациента.
- При изменении параметров, влияющих на расчет дозы, значение скорости всегда будет меняться, а значение дозировки будет скорректировано.
Рекомендации по применению
- Используйте только устойчивые к давлению линии (мин. 2 бар / 1500 mmHg).
- При подсоединении нескольких инфузионных линий к одному венозному доступу не исключается вероятность взаимного влияния линий.
- Касательно возможной несовместимости оборудования и лекарств, обратитесь к соответствующей информации производителей.
- Используйте только совместимые комбинации приборов, аксессуаров, рабочих компонентов и одноразовых принадлежностей с винтовым соединением.
- Использование несовместимых одноразовых принадлежностей может влиять на технические характеристики прибора.
- Подключаемые электроприборы должны соответствовать требованиям IEC/EN (напр. IEC/EN 60950 для оборудования по обработке данных). Пользователь несет всю ответственность за конфигурацию системы при подключении дополнительного медицинского оборудования. Должен быть принят во внимание международный стандарт IEC/EN 60601-1-1.
Список лекарств
До 720 наименований лекарств, включая параметры инфузии и информацию о лекарстве, могут быть сохранены в 15 категориях. Загрузка списка в насос может быть произведена с помощью отдельной компьютерной программы “Drug List Editor Space” (Редактор Списка Лекарств Спэйс).
Список лекарств доступен из Меню Пуск и Меню Специальные функции. Перед началом применения Списка, Пользователь должен убедиться, что Список лекарств в насосе соответствует данной группе пациентов. Наименование Списка лекарств будет отображено на экране насоса.
Существует несколько способов вызова Списка лекарств из Меню для последующего применения. Это возможно как во время инфузии, так и при остановке насоса.
С одной стороны, наименование лекарства со всеми параметрами инфузии может быть выбрано из Списка лекарств. С другой стороны, если скорость, объем и/или время уже были заданы в Главном меню, загружаются наименование лекарства и вновь заданные параметры инфузии. Если расчет дозы уже начат, последующее использование наименования лекарства из списка все равно возможно.
Блок данных
Функция Блок данных защищает прибор от несанкционированного доступа. Четырехзначный код (по умолчанию 9119), который может быть изменен через сервисную программу, активирует эту функцию. Существует три уровня защиты.
Уровень 1
Изменение значений, как и болюсная инфузия недоступны, но можно произвести смену шприца. Возможна навигация по разделам меню и проверка состояния насоса в меню Статус. Пуск, остановка и выключение насоса доступны.
Уровень 2
Этот уровень имеет те же характеристики, что и Уровень 1, но в дополнение к предыдущему, не позволяет произвести смену шприца. Для того, чтобы предотвратить активацию сигнала тревоги «Блок данных», необходимо ввести правильный код в течение 20 секунд после остановки насоса. Смена системы и выключение насоса возможны только после ввода правильного кода.
Уровень 3
Этот уровень позволяет начать и остановить инфузию, а также выключить насос. Код для этого уровня может быть различным для разных лекарств и устанавливается в Списке лекарств. Смена шприца, тем не менее, возможна с использованием кодов, установленных для двух предыдущих уровней.
Обзор различий между уровнями 1, 2 и 3 дан в таблице.
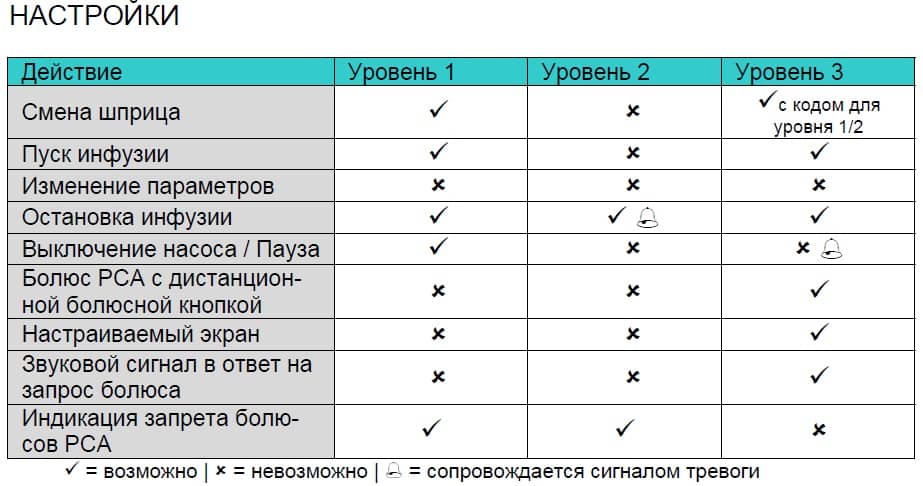
Сигналы и тревоги Perfusor Space B Braun
Перфузор Спэйс б браун оснащен звуковой и визуальной сигнализацией тревоги.
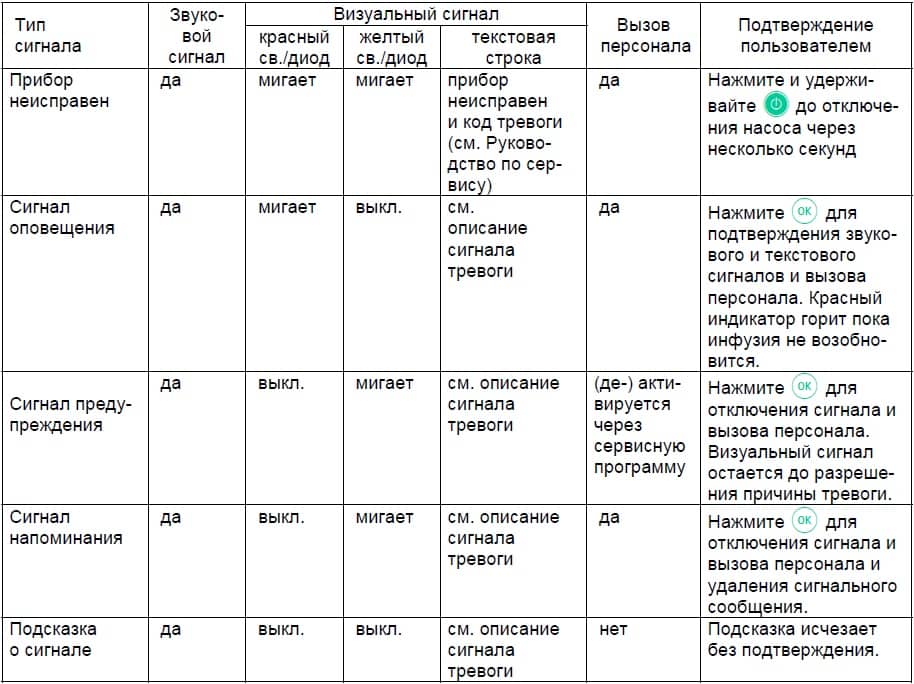
Сигналы неисправности Perfusor Space B Braun
При появлении сигнала неисправности прибора инфузия немедленно прекращается. Нажмите кнопку вкл/выкл для отключения прибора. Затем включите прибор снова. При повторном сигнале тревоги, отсоедините насос от пациента, откройте переднюю дверцу насоса и удалите шприц. Прибор необходимо передать в сервисную службу.
Сигналы предупреждения Perfusor Space B Braun
Сигналы предупреждения подаются за несколько минут (в зависимости от сервисных установок) перед сигналами оповещения. Сигнал предупреждения включает звуковой тон, мигающий желтый индикатор и активирует систему вызова персонала (опция). Текстовое сообщение зависит от причины тревоги. Звуковой тон и система вызова персонала отключаются нажатием кнопки «ОК». Экран и индикатор остаются в режиме предупреждения вплоть до отключения сигнала оповещения. Во время подачи сигнала предупреждения инфузия не прерывается.
Шприц почти введен
В шприце осталось очень мало жидкости.
Объем почти введен
Введение заданного объема близко к завершению.
Время истекает
Заданное время инфузии скоро истечет.
Батарея разряжается
Батарея почти разряжена.
KVO активен
Объем введен/Время истекло и насос продолжает инфузию в режиме KVO — Открытая вена.
Ошибка соединения
Насос установлен в систему, хотя бы один из приборов в которой несовместим или неисправен. Применение этого прибора в системе не разрешается. Систему необходимо передать для проверки в сервисную службу.
Таймер на экране ведет обратный отсчет оставшегося времени ( в зависимости от сервисной настройки, от 3 до 30 мин). После этого насос подает сигнал оповещения.
Сигналы оповещения Perfusor Space B Braun
Сигналы оповещения приводят к прерыванию инфузии. Подается звуковой сигнал, мигает красный индикатор и активируется система вызова персонала. На экране появляется сообщения «Тревога» и информация о причине сигнала тревоги. Звуковой сигнал и система вызова персонала могут быть отключены кнопкой «ОК». Коррекция должна быть произведена в соответствие с причиной сигнала тревоги.
Шприц пуст
В шприце не осталось жидкости. Из-за различий в допусках шприцев разных производителей, незначительное количество раствора в шприце может оставаться. Попытка продолжить инфузию приводит к полному опорожнению шприца и отключению через датчик давления. Выполните смену шприца.
Объем введен
Заданный объем введен. Продолжите инфузию или введите новые параметры.
Время истекло
Заданное время инфузии истекло. Продолжите инфузию или введите новые параметры.
Батарея разряжена
Батарея разряжена. Подключите прибор к сети и/или замените батарею. Сигнал о разрядке батареи длится 3 минуты, после этого насос автоматически отключается.
KVO остановлен
Время работы в режиме KVO истекло. Продолжите инфузию или введите новые параметры.
Высокое давление
Обнаружена окклюзия в системе. Достигнут установленный уровень давления. Насос автоматически снижает скорость введения. Проверьте отсутствие петель и перегибов инфузионной линии, проходимость инфузионного фильтра и в/в катетера. Увеличьте уровень окклюзионного давления, если необходимо. Из-за различия в допусках шприцев разных производителей, тревога по давлению может быть обусловлена высокой силой трения между поршнем и цилиндром.
Шприц установлен неверно
Упорные планки цилиндра и штока установлены неправильно.
Держатель шприца
Держатель шприца быль открыт во время инфузии. Закройте держатель шприца.
Крышка батарейного отсека удалена
Крышка батарейного отсека установлена неправильно. Переустановите крышку до щелчка.
Привод заблокирован
Внешние помехи препятствуют движению привода. Устраните все внешние помехи.
Откалибруйте прибор
Параметры калибровки насоса были изменены (например, после обновления программного обеспечения). Выполните калибровку через сервисную программу. Выполняется Сервисной службой.
Захват неисправен
Была нажата кнопка экстренного извлечения шприца и захваты были разведены вручную. Удалите шприц и обратитесь в Сервисную службу.
Планка штока не зафиксирована
Планка штока шприца не контактирует с датчиком давления насоса. Проверьте систему на наличие отрицательного давления и устраните причину.
Время паузы истекло
Установленное время паузы истекло. Задайте новое время паузы или возобновите предыдущую инфузию.
Батарея не установлена
Использование насоса без батареи невозможно. Отключите прибор и установите батарею.
Данные сброшены
Данные инфузии и насоса не возможно восстановить. Введите данные инфузии и настройки насоса заново.
Данные инфузии сброшены
Параметры инфузии не возможно восстановить. Введите параметры инфузии заново.
Блок данных
Была попытка остановить или отключить насос без ввода кода. Введите правильный код для соответствующего продолжения инфузии или выключите насос.
Красный индикатор не гаснет до возобновления инфузия или выключения насоса.
Если на экране появляется символ гаечного ключа и/или одновременно мигают желтый, красный и синий индикаторы – насос находится в сервисном режиме и его использование для лечения пациентов запрещено. Насос должен быть проверен сервисной службой.
Сигналы напоминания Perfusor Space B Braun
Сигналы напоминания подаются в двух случаях:
- Шприц установлен, инфузия не начата, значения не введены и прибор не работает в течение 2 минут. Включается звуковая сигнализация, мигает желтый индикатор и активируется система вызова персонала. Сообщение на экране «Сигнал напоминания!»; Сообщение на экране «Ввод параметров не завершен!». Для прекращения подачи сигнала тревоги нажмите «ОК» и продолжите ввод параметров инфузии и настроек насоса.
- Ввод данных начат, но не завершен или не подтвержден. Другая причина – шприц не установлен. Включается звуковая сигнализация, сообщение на экране «Значение не принято», мигает желтый индикатор и активируется система вызова персонала. Для прекращения подачи сигнала тревоги нажмите «ОК» и продолжите ввод параметров инфузии.
Подсказки о причинах сигналов тревоги
При некорректном вводе параметров, на экране появляются соответствующие подсказки, например «Внимание! Значение скорости превышает допустимое»; «Значение не может быть изменено». Включается звуковая сигнализация. Подсказки исчезают через несколько секунд и их подтверждение не требуется.
Работа от батареи и обслуживание
Перфузор Спэйс оснащен современной NiMH батареей. Время работы насоса с новой батареей составляет 8 часов при скорости инфузии 25 мл/ч. Для оптимальной работы аккумулятора, насос имеет защиту от перегрузки и полной разрядки. Батарея заряжается при включении прибора в сеть. При отключении от сети или в случае падения напряжения, насос автоматически переходит на питание от батареи.
Перед длительным хранением насоса (более 2-х недель без использования), батарея должна быть полностью заряжена, а затем извлечена из насоса. Перед извлечением (сменой) батареи всегда отсоединяйте насос от пациента и отключайте прибор.
Индикатор заряда батареи отображается на экране (низкий, средний, полный заряд). Для получения более детальной информации о состоянии батареи (время работы в часах и минутах) необходимо в меню «Статус» войти в раздел «Батарея».
Самотестирование батареи Perfusor Space B Braun
Если символ батареи мигает во время работы от сети, батарея либо разряжена, либо быстро разряжается. В этом случае насос не должен отключаться от сети. Если необходимо экстренно отключить насос от сети, убедитесь, что остаточный заряд батареи достаточен для применения. Если символ батареи мигает непрерывно (>1ч), батарея должна быть проверена техническим персоналом и замене-на при необходимости.
Оптимальное использование батареи
Факторы, влияющие на срок службы батареи:
- окружающая среда;
- меняющаяся нагрузка (напр. частая подача болюсов).
Срок службы батареи можно увеличить путем ее регулярной зарядки и разрядки. Для этого насос должен работать от батареи до появления сигнала тревоги о ее разрядке. После этого прибор необходимо подключить к сети минимум на 6 часов. Рекомендуется выполнять данную процедуру один раз в месяц.
Кроме того:
- Если возможно, заряжайте батарею только после ее полной разрядки.
- Если батарея, которая не разряжена полностью, заряжается несколько раз, ее емкость может уменьшиться. Первоначальная емкость может быть восстановлена при полной разрядке и последующей зарядке батареи.
- При нормальных температурных условиях батарея может быть заряжена и разряжена приблизительно 500 раз, после чего длительность ее работы снижается.
- При отключении насоса от сети батарея медленно разряжается. Это происходит даже, когда насос не работает. Первоначальная емкость может быть достигнута после нескольких циклов зарядки и разрядки.
- Время работы батареи может быть определено только при непрерывной работе насоса с полностью заряженной батареей при комнатной температуре. Отображаемое на экране насоса приблизительное время работы батареи основывается на текущей скорости инфузии. При длительном сроке использования, реальное время работы батареи снижается.
Батареи могут взрываться и протекать при вскрытии или сжигании. Соблюдайте правила утилизации!
Обслуживание батареи Perfusor Space B Braun
Для точной регулировки емкости батареи необходимо ее циклическое обслуживание. Насос запрашивает Пользователя о проведении обслуживания батареи каждые 30 дней. В режиме обслуживания батареи определяется возможная потеря емкости (например, из-за старения батареи) и затем емкость и время работы от батареи пересчитываются заново. После длительного хранения или длительной работы без обслуживания батареи, может случиться так, что время подачи предупредительного сигнала больше не будет поддерживаться. В этом случае необходимо проведение обслуживания батареи.
Для инициализации полной разрядки батареи на экране появляется запрос «Обслуживание батареи» и отображается кнопка «ОК». Для того, чтобы запустить процесс разрядки батареи, нажмите «ОК» и «вверх». При включении насоса процесс прерывается. Если обслуживание батареи необходимо продолжить, нужно повторно активировать режим обслуживания. После полной разрядки батареи происходит ее полная зарядка. Полное обслуживание батареи длится приблизительно двенадцать часов.
Примите во внимание, что время работы от батареи может быть меньше, если обслуживание батареи не завершено.
Технические характеристики Perfusor Space B Braun
Ниже указаны технические характеристики насоса Perfusor Space B.Braun.
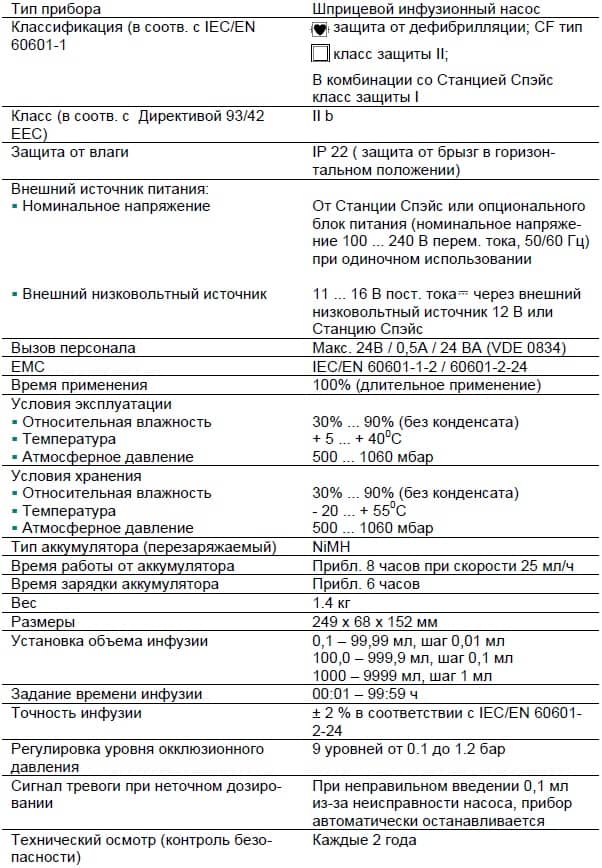
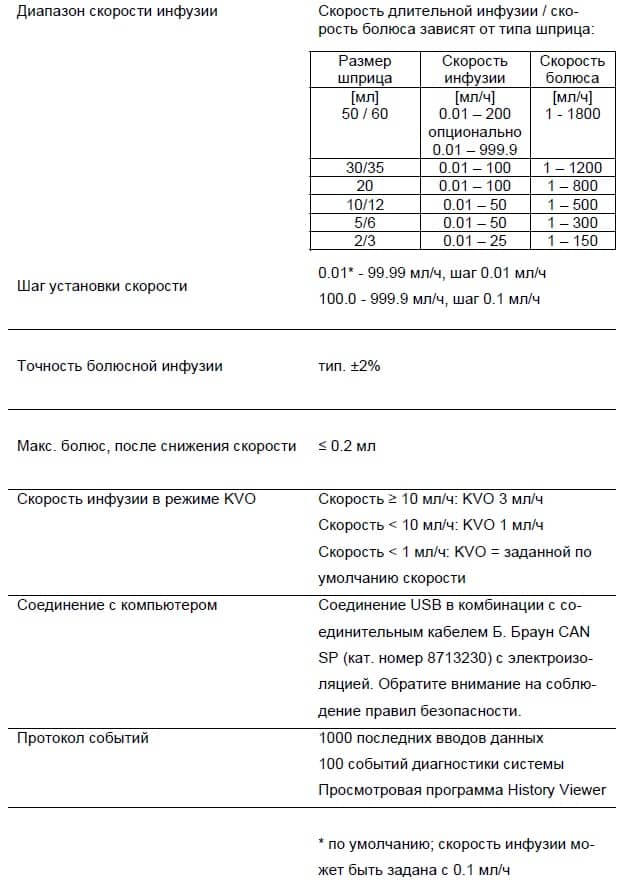
Гарантия Perfusor Space B Braun
Компания Б. Браун предоставляет 24 месяца гарантии, с момента поставки на каждый Перфузор Спэйс (12 месяцев на каждый аккумулятор (Battery Pack SP)). Гарантия предусматривает ремонт или замену отдельных частей, вышедших из строя в результате конструкторских или производственных ошибок, а так же дефектов материала. Срок действия гарантии прекращается в случае модернизации или ремонта, проведенных Пользователем или посторонними лицами.
Гарантия не распространяется на устранение дефектов, вызванных неправильным / неумелым обращением или нормальным износом прибора.
Регулярная проверка Perfusor Space B Braun
Проверяйте чистоту, комплектность, отсутствие повреждений. Используйте прибор только согласно Руководству по применению. При каждом включении проверьте: самотестирование, звуковой сигнал, индикацию работы и сигнализацию.
Уход Perfusor Space B Braun
Очищайте поверхность насоса с использованием мыльного раствора. Не рекомендуется применять распылители в местах контакта с электричеством. Рекомендуется: средство для протирания, например Мелисептол фирмы Б. Браун. После очистки прибор должен высохнуть в течение как минимум одной минуты. Не распыляйте в отверстия прибора. Соблюдайте инструкции по утилизации использованных расходных материалов и уходу за батареями и принадлежностями. Увеличительное стекло передней крышки и экран протирайте мягкой салфеткой. Не используйте Гексакварт.
Принадлежности и каталожные номера Perfusor Space B Braun
Б.Браун Перфузор Спэйс (871 3030)
Станция Спэйс (871 3140)
Станция Спэйс предназначена для установки до четырех насосов.
Верхняя крышка СпэйсКавер стандарт (871 3147)
Верхняя крышка СпэйсКавер комфорт (871 3145)
Верхняя крышка предназначена для установки на Станцию Спэйс. Крышка имеет встроенную ручку для переноски Станции. СпэйсКавер комфорт дополнительно оснащена центральной сигнализацией с блоком управления и сигнальными индикаторами.
Универсальный зажим Спэйс (871 3130)
Предназначен для фиксации до трех насосов Б. Браун Спэйс и одного модуля Спэйс Контроль и их стыковки в единый блок.
Блок питания Спэйс (871 3110A)
Обеспечивает питание от сети для одного насоса или для модуля Спэйс Контроль.
Комби — кабель Спэйс 12 В (871 3133)
Комби – кабель Спэйс позволяет объединить до трех насосов. Все насосы могут быть подключены через блок питания Спэйс или соединительный кабель 12 В.
Перезаряжаемая батарея Спэйс (871 3180)
Интерфейсный кабель CAN Спэйс (871 3230)
Интерфейсный кабель CAN Спэйс требуется для соединения Станции Спэйс / насоса Спэйс с компьютером (для сервисных целей).
Соединительный кабель Спэйс 12 В (871 3231)
Кабель Спэйс для системы вызова персонала (871 3232)
Используйте указанный соединительный кабель для подключения насоса Перфузор Спэйс к системе вызова персонала. Подключение к системе вызова персонала необходимо для выполнения требований VDE 0834.
Оригинальные шприцы Perfusor Space B Braun
- Шприц Перфузор 50 мл без иглы, ЛЛ (872 8844F);
- Шприц Перфузор 50 мл c иглой, ЛЛ (872 8810F);
- Шприц Перфузор 50 мл c иглой и фильтром, ЛЛ (872 8852F);
- Шприц Перфузор 50 мл c иглой и фильтром, светозащитный, ЛЛ (872 8828F);
- Шприц Перфузор 20 мл без иглы, ЛЛ (872 8615);
- Шприц Перфузор 20 мл c иглой, ЛЛ (872 8623);
- Шприц Омнификс 50/60 мл, ЛЛ (461 7509F);
- Шприц Омнификс 30 мл, ЛЛ (461 7304F);
- Шприц Омнификс 20 мл, ЛЛ (461 7207V);
- Шприц Омнификс 10 мл, ЛЛ (461 7100V);
- Шприц Омнификс 5 мл, ЛЛ (461 7053V);
- Шприц Омнификс 2 мл, ЛЛ (461 7029V).
Оригинальные линии Perfusor Space B Braun
- Линия Перфузор, ПВХ, 50 см (825 5172);
- Линия Перфузор, ПВХ, 150 см (872 2960);
- Линия Перфузор, ПВХ, 200 см (872 2862);
- Линия Перфузор, ПВХ, 250 см (825 5490);
- Линия Перфузор, ПВХ, 300 см (825 5253);
- Линия Перфузор, ПЭ, 50 см (825 5059);
- Линия Перфузор, ПЭ, 100 см (825 5067);
- Линия Перфузор, ПЭ, 150 см (872 2935);
- Линия Перфузор, ПЭ, 200 см (872 3060);
- Линия Перфузор, ПЭ, 250 см (827 2565);
- Линия Перфузор, ПВХ, 150 см, безыгольный коннектор Сэйфсайт (872 2820);
- Линия Перфузор, ПВХ, 200 см, инъекционный фильтр 0,22 мкм (872 3001);
- Линия Перфузор, ПВХ, 168 см, PCA, с возвратным клапаном (872 6019);
- Линия Перфузор, тип МР, ПВХ, 75 см, с перекидной гайкой (872 2870);
- Линия Перфузор, тип МР, ПВХ, 150 см, с перекидной гайкой (825 5504);
- Линия Перфузор, ПЭ, 150 см, светозащитная (872 3010).
Скачать инструкцию на Perfusor Space B Braun
Скачать инструкцию и другую документацию на Perfusor Space B Braun можно здесь.
Руководство пользователя ( user manual ) на русском языке Perfusor Space B Braun скачать.
Сервисная инструкция ( service manual ) на английском языке Perfusor Space B Braun скачать.
Регистрационное удостоверение Perfusor Space B Braun скачать.
Так же смотрите Space Control, Инфузионный насос DI 2000 Medifusion, Инфузионный насос Aitecs DF-12M.

- Manuals
- Brands
- B. Braun Manuals
- Medical Equipment
- Perfusor Space
- Service manual
-
Contents
-
Table of Contents
-
Troubleshooting
-
Bookmarks
Quick Links
1.0
1.3
Perfusor® Space
Service Manual
Version 1.3 English
Related Manuals for B. Braun Perfusor Space
Summary of Contents for B. Braun Perfusor Space
-
Page 1
Perfusor® Space Service Manual Version 1.3 English… -
Page 2
This Service Manual is valid for: Designation Part No. Infusion syringe pump Perfusor® Space … . 0871 3030 This Service Manual is available under Designation Part No. the following part number: Service Manual Perfusor®… -
Page 3
Table of Contents Important Preliminary Remarks Service Work Page 0 — 5 Technical Safety Checks Page 0 — 5 Current Versions Page 0 — 5 Revision Service Page 0 — 5 Quality Management Page 0 — 6 Checks and Repair Page 0 — 6 Notes on ESD… -
Page 4
Table of Contents Servicing the Unit Cleaning Page 4 — 1 Servicing the Battery Page 4 — 1 Technical Safety Check (TSC) Perfusor® Space 1 Technical Safety Check (TSC) Power Supply SP 1 Procedural Instructions on the TSC Visual Inspection Page 7 — 1 Electrical Safety… -
Page 5
You will be included in the revision service after: technical training by B. Braun Melsungen or a written order placed with the sales department of B. Braun (fee required). Perfusor® Space, 1.1 gb 0 — 5… -
Page 6
B. Braun unit. Quality Management B. Braun is certified in accordance with DIN EN ISO 9001 and ISO 13485. This certification also includes maintenance and service. The unit has the CE label. The CE label confirms that the device corresponds to the “Directive of the Council for Medical Products… -
Page 7
Service personnel are responsible for the calibration of their test equipment. Original test equipment can be calibrated at the works of B. Braun. Further information is available upon request. Setting Off Additional notes and warnings are set off as follows:… -
Page 8
Important Preliminary Remarks References to chapters are shown as follows (see “Setting Off“ pg. 0 — 7) References to figures and tables are shown as follows Fig.: 2 — 3 Table 2 — 1 References to item numbers in figures are shown as follows (Fig.: 1 — 1 / Item In this case “Fig.: 1 — 1“… -
Page 9
Important Preliminary Remarks List of Abbreviations Abbreviations which are not generally known, but are used in this manual, are listed below. Controller Area Network Communauté Européenne (European Communities) Calibration Step Deutsche Industrie Norm (German Industrial Standard) European Standard Electrostatic Discharge Function Microprocessor International Electrotechnical Commission… -
Page 10
Important Preliminary Remarks Unit Test Step Verband der Elektrotechnik, Elektronik und Informationstechnik e.V. (German electrical engineering association) 0 — 10 Perfusor® Space, 1.2 gb… -
Page 11
Entry for Technical Training Application for a technical training course must be made via the responsible representative. Ordering of Spare Parts and Test Equipment Please contact your local B. Braun subsidary. International Technicians (Intercompany) Nadja Machal Fax: +49 5661 / 75 — 47 89 e-mail: nadja.machal@bbraun.com… -
Page 12
Contact Persons For your notes: 0 — 12 Perfusor® Space, 1.0 gb… -
Page 13
System Overview Description The Perfusor® Space (PSP) is according to IEC/EN 60601 resp. IEC/ EN 60601-2-24 a transportable infusion syringe pump for administrating fluids in the nutritional therapy and infusion technique as well as for home care applications. The medical specialist must decide on suitability for application on the basis of the warranted properties and the technical data. -
Page 14
System Overview Physical Construction Fig.: 1 — 2 Perfusor® Space Legend of fig. 1 — 2: ItemDesignation Perfusor® Space Connector “P2“ for SpaceStation module, external 12 V DC and accessories Drive head Connector “P3“, connection to SpaceControl module Syringe holder with piston brake Battery compartment cover Operating Unit Syringe area… -
Page 15
System Overview The Perfusor® Space housing mainly consists of the bottom part and the upper part. The battery module is inserted in the rear of the housing upper part. The opening is covered by the battery compartment cover. The operating unit is attached to the front of the bottom part with two hinges. -
Page 16
System Overview The pressure in the infusion system is measured through a strain gauge measuring in the drive head and monitored in the device electronics. The data from the strain gauge is continuously compared with the limit values which are calculated dependent on the selected syringe type and the pressure settings. -
Page 17
System Overview Fig.: 1 — 3 Block diagram Perfusor® Space Perfusor® Space, 1.0 gb 1 — 5… -
Page 18
System Overview Unit Software Approved Software Versions 688A030032 Basic software Position 1 2 3 4 5 6 7 8 9 10 688A030035 Digit 6 8 8 C 0 3 0 0 0 1 Improved functions 688A030040 Revision level Improved functions Hardware Languages French and Swedish added Software group… -
Page 19
PC is switched off, a component of the software may be seriously damaged so that repairs are no longer possible. In such a case the software cannot be updated via the PC and the device must be returned to B. Braun. Service Program Approved Version… -
Page 20
System Overview 1. Start the “HiBaSeD.exe” program (History, Barcode, Service, Drug list) on the PC. The Service Program is loaded and started and the initial window of the Service Program is displayed. 2. Read the notes carefully. 3. Mark the field “I accept all conditions” and then the field “Yes”… -
Page 21
System Overview The work window of the Service Program appears on the screen. All devices recognized are listed in the left column. Fig.: 1 — 8 5. Activate the desired device from the list on the left in the work window with a double-click. The device data is then displayed below the device name. -
Page 22
System Overview If the unit software version is not compatible with the Service Program version, a window opens prompting the operator to change the Service Program version. This window displays a compatibility list of the Service Program- and unit software versions. -
Page 23
System Overview Service Program Version Help Info … 1. Open the “HiBaSeD“ window via . The current version of the Service Program is shown in this window. 2. Close the window by clicking “OK”. Fig.: 1 — 11 Compatibility List Help 1. -
Page 24
System Overview Technical Data All technical data is indicated in the instructions for use. Options The functions of the individual options are detailed in the instructions for use. Perfusor® Space Designation Part No.: Power supply Euro ……0871 3110A Power supply UK. -
Page 25
Unit Diagnosis / Calibration General WARNING WHILE TESTING THE UNIT AND TROUBLE SHOOTING THE OPERATOR/SERVICE TECHNICIAN MUST WORK WITH VOLTAGES UP TO 115 / 230 V AC. THESE VOLTAGES MAY CAUSE INJURIES WHICH ARE DANGEROUS TO LIFE AND LIMB. THE NATIONAL AND INTERNATIONAL SAFETY REGULATIONS ARE TO BE ADHERED TO. -
Page 26
Unit Diagnosis / Calibration The unit check, calibration and trouble shooting are subdivided into numbered working steps (Unit Test Step UTS, Calibration Step CS, Trouble Shooting TS) and are based on each other. Beginning with UTS 1 the operation described here has to be executed. -
Page 27
Unit Diagnosis / Calibration Alarms and Error Codes The alarms of the Perfusor® Space are classified in 5 categories. These categories are listed hereafter according to their importance. Alarm advice In case of unacceptable inputs corresponding messages are displayed (e.g. “Caution! Rate out of range“, “The parameter cannot be changed“) and a beep sounds. -
Page 28
Unit Diagnosis / Calibration Alarms Alarm Possible Cause Fault Clearance Battery nearly discharged (type: pre- The device was not connected to the Operate the device with battery until the alarm) mains long enough message “Battery discharged“ is displayed and the unit is switched off. Then connect the unit to the mains for at least 6 hours. -
Page 29
Unit Diagnosis / Calibration Device Alarms of the Function Processor Error Code Definition Possible Cause Fault Clearance 1001 … 1013 Internal Error 1014 Loudspeaker not off Loudspeaker connector Check the loudspeaker connector Loudspeaker Check the loudspeaker 1015 Loudspeaker lost Loudspeaker connector Check the loudspeaker connector Loudspeaker Check the loudspeaker… -
Page 30
Unit Diagnosis / Calibration Device Alarms of the Control Microprocessor Error Code Definition Possible Cause Fault Clearance 1100 Timebase too fast Quartz of the processor PCB Exchange processor PCB 1101 Timebase too slow Quartz of the processor PCB Exchange processor PCB 1102 Timebase fail Quartz of the processor PCB… -
Page 31
Unit Diagnosis / Calibration Error Code Definition Possible Cause Fault Clearance 1201 different version FuP to KuP Software Update unit software Software 1202 E_ERROR_STEPMOTOR_1 Phase Drive motor, lead screw Exchange processor PCB not ok 1203 E_ERROR_STEPMOTOR_2 Current Motor drive Carry out calibration value not 0x55 Recognition of direction of rotation… -
Page 32
Unit Diagnosis / Calibration The Most Important Error Modes The following list specifies the most important error modes and their clearance. Note The device must be checked after every repair or service (see “Device Check“ pg. 2 — Error Possible Cause Fault Clearance The battery module discharges too fast The device was not used for a longer time. -
Page 33
Unit Diagnosis / Calibration Device Check Activity Function If yes If no The device is inserted in a SpaceStation or UTS 2 UTS 3 connected to a SpaceControl. Remove the device. UTS 4 Loosen all connections from the device. UTS 4 Remove syringe and close syringe holder. -
Page 34
Unit Diagnosis / Calibration Activity Function If yes If no Insert 2 ml / 3 ml syringe. On the LC display “Brake: stopped by holder” UTS 22 TS 21 appears in the line. Open syringe holder and remove syringe. On the LC display “Brake: stopped by light barrier” UTS 23 TS 21 appears in the line. -
Page 35
Unit Diagnosis / Calibration Activity Function If yes If no Insert syringe gauge for the strain gauge UTS 41 measurement, close syringe holder and select syringe type „#Lehre OPS50“. The syringe gauge must not be tipped. Therefore fix the syringe gauge so far into the syringe recess by hand that the piston brake moves back and the claws surrounds the pressure element. -
Page 36
Unit Diagnosis / Calibration Activity Function If yes If no Confirm syringe change, release syringe gauge and UTS 48 remove gauge. WARNING WHILE CHECKING THE MOTOR POWER LIMITATION WITH THE SYRINGE GAUGE THE SYRINGE HOLDER MUST NOT BE OPENED. THE SYRINGE GAUGE IS UNDER VERY HIGH PRESSURE AND MAY CAUSE INJURIES IF THE PRESSURE IS RELIEVED SUDDENLY. -
Page 37
Unit Diagnosis / Calibration Activity Function If yes If no Select pressure stage 6 and start infusion. When the maximum pressure of this pressure stage UTS 56 CS 1 is reached, the delivery is stopped, the red LED on the operating unit flashes and the message “Alarm / Drive blocked”… -
Page 38
Unit Diagnosis / Calibration Calibration Activity Function If yes If no Connect unit to PC with interface cable. CS 2 Start Service Program on the PC (see “Starting the The desired device is found by the Service Program CS 3 Service Program“… -
Page 39
Unit Diagnosis / Calibration 1. Start the Service Program (see “Starting the Service Program“ pg. 1 — 2. Select the unit to be calibrated in the left column of the window with a double mouse-click. The blue and the yellow LED blinks in opposite with the red LED. -
Page 40
Unit Diagnosis / Calibration 5. Input your user number in the window “Worker ID” as well as the six-digit serial number of the device, if necessary. 6. Confirm the input with “OK”. Fig.: 2 — 3 Note If HiBaSeD could not clearly read the device serial number, the number must be entered according to the rating plate. -
Page 41
Unit Diagnosis / Calibration Fig.: 2 — 6 Perfusor® Space, 1.2 gb 2 — 17… -
Page 42
Unit Diagnosis / Calibration 8. The frame “Data selection” is now activated. Fig.: 2 — 7 If the “Modify” button was pressed in the “Calibration procedure” frame, the desired data for editing and transmission can now be selected in the “Data selection” frame. -
Page 43
Unit Diagnosis / Calibration 9. Mark at least the “Calibration data“ field in this frame if you have not selected “New device” and confirm by clicking “OK”. The device switches on and the drive head moves to the extended end position. You are prompted to press the blue connection key on the device. -
Page 44
Unit Diagnosis / Calibration Fig.: 2 — 9 11. In the frame “Type of calibration” you can choose between a complete or a partial calibration. Select the desired calibration mode with the mouse pointer. Note The following description is applicable to a complete and a partial calibration. -
Page 45
Unit Diagnosis / Calibration 12. Press the “OK“ button after you have selected the calibration elements. The necessary device data is read out and stored in the PC. Fig.: 2 — 10 Fig.: 2 — 11 Fig.: 2 — 12 Fig.: 2 — 13 Perfusor®… -
Page 46
Unit Diagnosis / Calibration Fig.: 2 — 14 13. The frame “Element selection” is now activated. If you have selected a complete calibration in the “Type of calibration” frame, the individual calibration elements are already selected and cannot be changed. Actuate the “OK” button. -
Page 47
Unit Diagnosis / Calibration Claw Calibration 1. The frame “Claw calibration” is activated and calibration is started. 2. The claws in the drive head are closed and the query “Claw closed? Please confirm” is displayed in the frame “Claw calibration”. 3. -
Page 48
Unit Diagnosis / Calibration 4. Insert diameter gauge 23.4 mm and close the syringe holder. 5. Press the “OK” button. Fig.: 2 — 18 6. Insert diameter gauge 15.7 mm and close the syringe holder. 7. Press the “OK” button. Fig.: 2 — 19 2 — 24 Perfusor®… -
Page 49
Unit Diagnosis / Calibration 8. Insert diameter gauge 9.0 mm and close the syringe holder. 9. Press the “OK” button. Fig.: 2 — 20 10. Open the syringe holder and remove the diameter gauge. Press the “OK” button. Note The syringe holder must always be completely turned and the axial fastening completely opened. -
Page 50
Unit Diagnosis / Calibration 11. Close the syringe holder and actuate the “OK” button. If calibration was not terminated successfully, an error message is displayed on the PC screen. Fig.: 2 — 22 Pressure Calibration Note The term “Power gauge” in the windows of the Service Program corresponds to the syringe gauge. -
Page 51
Unit Diagnosis / Calibration 4. If “Insert power gauge and confirm with OK” is displayed in the frame “Pressure calibration/length/PWM”, open the syringe holder and remove the length gauge. WARNING DURING PRESSURE CALIBRATION WITH THE SYRINGE GAUGE THE SYRINGE HOLDER MUST NOT BE OPENED. THE SYRINGE GAUGE IS UNDER VERY HIGH PRESSURE AND MAY CAUSE INJURIES IF THE PRESSURE IS RELIEVED SUDDENLY. -
Page 52
Unit Diagnosis / Calibration This report can be printed out by pressing the “Print” button. Fig.: 2 — 27 2. Actuate the “OK” button to finish the calibration process and to store the data in the device. 2 — 28 Perfusor®… -
Page 53
Unit Diagnosis / Calibration Trouble Shooting Note The following trouble shooting cannot be carried out independently. It is based on the precise observance of the steps for the device check (see “Device Check“ pg. 2 — 9). From there reference is made to the corresponding trouble shooting steps. Activity Function If yes… -
Page 54
Unit Diagnosis / Calibration Activity Function If yes If no Switch on unit and insert syringe. The syringe piston is fastened with the syringe UTS 19 TS 23 holder blade and the default message is displayed on the LC display. Exchange processor PCB. -
Page 55
Disassembly / Assembly Perfusor® Space 3.1 General Remarks on Disassembly / Assembly Before disassembling the unit, the system must be checked (see “Device Check“ pg. 2 — 9) to isolate the part to be exchanged. The necessary steps to disassemble the complete unit, all its subsystems and spare parts are detailed in the following description. -
Page 56
Disassembly / Assembly Preparations for Exchanging the Processor PCB If the processor PCB is to be replaced a back-up of the pump settings is to be carried out, if this is still possible. 1. Start the Service Program (see “Starting the Service Program“ pg. -
Page 57
Disassembly / Assembly 4. Select the tab “IO”. 5. Press the “To file” button. In the window which opens now you are asked for the storage position of the file on the PC hard disk and the file name. Fig.: 3 — 2 6. -
Page 58
Disassembly / Assembly 7. Actuate the “Display” button to display the device data on screen. With “Close” the window is closed again. Fig.: 3 — 3 8. Select the storage position in the “Save as” window and input a unique file name. 9. -
Page 59
Disassembly / Assembly 10. Select the tab “Disposable articles”. Fig.: 3 — 5 11. Actuate the “From device” button. The data is read from the pump. The data of the disposable articles read out is displayed on screen. Perfusor® Space, 1.2 gb 3 — 5… -
Page 60
Disassembly / Assembly Fig.: 3 — 6 12. Press the “Save as” button. In the window which opens now you are asked for the storage position of the file on the PC hard disk and the file name. 3 — 6 Perfusor®… -
Page 61
Disassembly / Assembly 13. Select the storage position in the “Save as” window and input a unique file name. 14. Press the “Save” button. The data of the pump is saved on the PC hard disk. 15. Exit the Service Program (see “Quit the Service Program“… -
Page 62
Disassembly / Assembly Service Parts and Screw Kit All small parts, such as cover caps, are contained in a Perfusor® Space service part kit. Designation Ord. No. Service part kit Perfusor® Space ….3477 4270 with: housing cover cap (40 pieces) cover caps for operating unit (10 pieces) -
Page 63
Disassembly / Assembly 3.2 Battery Module Designation Ord. No. Battery compartment cover PSP , cpl….3452 0872 Battery pack SP (NIMH)……0871 3180 Disassembly Note Move the drive head to the extended end position before starting… -
Page 64
Disassembly / Assembly 2. Lift the lock (Fig.: 3 — 9 / Item 3) on the battery pack (Fig.: 3 — 9 / Item 1) and remove the battery pack out of the device. Fig.: 3 — 9 Legend of fig. 3 — 9: ItemDesignation Battery pack Battery compartment cover… -
Page 65
Disassembly / Assembly 3.3 Unit Foot Designation Ord. No. Unit foot (see “Service Parts and Screw Kit“ pg. 3 — Disassembly 1. Pull the unit foot (Fig.: 3 — 10 / Item 1) out of the housing. Fig.: 3 — 10 Legend of fig.
Disassembly 1. Pull the unit foot (Fig.: 3 — 10 / Item 1) out of the housing. Fig.: 3 — 10 Legend of fig. -
Page 66
Disassembly / Assembly 3.4 Operating Unit Designation Ord. No. Operating unit PSP, cpl……3452 0970 Hinge plate PSP, left ……3452 1011 Hinge plate PSP, right . -
Page 67
Disassembly / Assembly 4. Push the right and left connector locks (Fig.: 3 — 12 / Item carefully forward. 5. Disconnect the operating unit connection cable (Fig.: 3 — 12 / Item 1) from the connector. Fig.: 3 — 12 Legend of fig. -
Page 68
Disassembly / Assembly 6. Pull the operating unit from the left hinge plate (Fig.: 3 — 13 / Item 7. Pierce two cover caps (Fig.: 3 — 13 / Item 1) with a small screwdriver and remove cover caps. 8. Unscrew two screws and remove the left hinge plate. Fig.: 3 — 13 Legend of fig. -
Page 69
Disassembly / Assembly Disassembly 1. Pierce six cover caps (Fig.: 3 — 14 / Item 1) with a small screwdriver and remove cover caps. 2. Unscrew six screws and remove the rear panel (Fig.: 3 — 14 / Item Note The three screws of the keyboard must not be loosened. -
Page 70
Disassembly / Assembly 3. Push the right and left connector locks of the PCB keyboard carefully to the left. 4. Pull the LC display ribbon cable (Fig.: 3 — 16 / Item 1) out of the connector. Fig.: 3 — 15 Legend of fig. -
Page 71
Disassembly / Assembly 3.5 Upper Part of Housing Designation Ord. No. Upper part of housing PSP ….. . 3452 0910 Screws and cover caps (see “Service Parts and Screw Kit“… -
Page 72
Disassembly / Assembly 4. Unscrew two screws and remove the contact strip (Fig.: 3 — 18 / Item (Fig.: 3 — 18 / Item Note If necessary, the spring-mounted contact pins must be carefully inserted when the contact strip is dismounted. Fig.: 3 — 18 Legend of fig. -
Page 73
Disassembly / Assembly 3.6 Release Button Designation Ord. No. Release button PSP with leaf spring (see “Service Parts and Screw Kit“ pg. 3 — Disassembly 1. Pull the release button (Fig.: 3 — 19 / Item 1) out of the housing bottom part.
Disassembly 1. Pull the release button (Fig.: 3 — 19 / Item 1) out of the housing bottom part. -
Page 74
Disassembly / Assembly 3.8 Drive Designation Ord. No. Drive PSP, cpl. Silver claws ……. 3452 1046 Green claws (as from unit software “F”) . -
Page 75
Disassembly / Assembly Fig.: 3 — 23 Legend of fig. 3 — 23: ItemDesignation Screw EJOT 22×8 WN 5451 TORX 6IP A2 Washer Fastening angle Carrier Screw EJOT 22×8 WN 5451 TORX 6IP A2 Drive head Perfusor® Space, 1.1 gb 3 — 21… -
Page 76
Disassembly / Assembly 6. Pull the drive motor connector (Fig.: 3 — 24 / Item 2) off the processor PCB. 7. Unscrew one screw (first temporary screw) and remove the processor PCB ground cable (Fig.: 3 — 24 / Item Fig.: 3 — 24 Legend of fig. -
Page 77
Disassembly / Assembly 8. Unscrew one screw (second temporary screw) and take the drive assembly (Fig.: 3 — 25 / Item 1) out of the housing. Note Please pay attention to the corresponding notes during assembly and installation (see “Assembly / Installation“ pg. -
Page 78
Disassembly / Assembly Disassembly of the Drive Head 1. Pierce two cover caps (Fig.: 3 — 27 / Item 4) with a small screwdriver and remove cover caps. 2. Unscrew two screws (Fig.: 3 — 27 / Item 3. Remove claws (Fig.: 3 — 27 / Item 2) carefully out of the drive head. -
Page 79
Disassembly / Assembly 7. Pull the claw mechanism (Fig.: 3 — 29 / Item 3) off the drive head cover (Fig.: 3 — 29 / Item 2) until the connection cable connector (Fig.: 3 — 30 / Item (Fig.: 3 — 29 / Item 1) can be easily accessed. -
Page 80
Disassembly / Assembly 10. Unscrew one screw (Fig.: 3 — 31 / Item 2) and remove the ground wire (Fig.: 3 — 31 / Item 1) of the drive PCB (Fig.: 3 — 31 / Item 3) from the driving tube. CAUTION Pay attention to the strain gauge (Fig.: 3 — 31 / Item… -
Page 81
Disassembly / Assembly 3.9 Syringe Holder with Piston Brake Designation Ord. No. Piston brake PSP ribbon cable….3452 0864 Syringe holder with piston brake PSP, cpl… 3452 0945 Syringe holder PSP spring . -
Page 82
Disassembly / Assembly 3. Unscrew one screw and remove the locking clip (Fig.: 3 — 33 / Item 2) from the piston brake PCB. Fig.: 3 — 33 Legend of fig. 3 — 33: ItemDesignation Screw EJOT 20×14 WN 5452 TORX 6IP Locking clip 3 — 28 Perfusor®… -
Page 83
Disassembly / Assembly 4. Open the right and left connector lock (Fig.: 3 — 34 / Item on the piston brake PCB carefully. Fig.: 3 — 34 Legend of fig. 3 — 34: ItemDesignation Piston brake connector lock 5. Pull the ribbon cable (Fig.: 3 — 35 / Item 1) out of the connector and remove the cable from the housing. -
Page 84
Disassembly / Assembly 6. Remove the syringe holder spring (Fig.: 3 — 36 / Item 1) from the post in the bottom part of the housing. Note The syringe holder spring can only be removed together with the piston brake drive. Fig.: 3 — 36 Legend of fig. -
Page 85
Disassembly / Assembly 11. Drive the piston brake motor with maximum 3 V DC. The blade is moved out of the syringe holder housing. Note If the blade moves into the syringe holder housing, then the polarity on the piston brake motor must be changed. 12. -
Page 86
Disassembly / Assembly 14. Unscrew one screw and remove the bearing cover (Fig.: 3 — 40 / Item 15. Take the lock (Fig.: 3 — 40 / Item 3) out of the bearing housing. Fig.: 3 — 40 Legend of fig. 3 — 40: ItemDesignation Screw EJOT 20×12 WN 5452 TORX 6IP Bearing cover… -
Page 87
Disassembly / Assembly 18. Unscrew two screws and remove guide rail (Fig.: 3 — 42 / Item 2) out of the bottom part of the housing. 19. Take piston brake motor assembly (Fig.: 3 — 42 / Item 4) out of the housing bottom part. -
Page 88
Disassembly / Assembly 3.10 Processor PCB Designation Ord. No. Processor PCB PSP ……3452 0880 (incl. -
Page 89
Disassembly / Assembly 3.11 Assembly / Installation Assembly or installation of the modules and subsystems is done in reverse order of disassembly. Special steps to be observed are described hereafter in detail. Only new cover caps are to be used. Special Screws Special screws for plastic housings are used in this unit. -
Page 90
Disassembly / Assembly Processor PCB When the processor PCB is replaced all data of the pump except for the calibration data was probably saved on a PC (see “Preparations for Exchanging the Processor PCB“ pg. 3 — Carry out the following steps to transmit the data back to the device. -
Page 91
Disassembly / Assembly Fig.: 3 — 44 4. Select the desired file with the mouse pointer and press the “Open” button. Fig.: 3 — 45 Perfusor® Space, 1.2 gb 3 — 37… -
Page 92
Disassembly / Assembly 5. Change to the tab “IO” and actuate the “To device” button. Fig.: 3 — 46 6. Press the “OK” button when the window “Confirmation” is displayed. Fig.: 3 — 47 3 — 38 Perfusor® Space, 1.2 gb… -
Page 93
Disassembly / Assembly 7. Enter your worker id in the window “Worker ID”. Press the “OK” button to transmit the data to the device. Fig.: 3 — 48 A message is displayed that a change of the device data may affect the patients. -
Page 94
Disassembly / Assembly 8. Select the tab “Disposable articles”. Fig.: 3 — 50 9. Press the “Load from“ button. The window “Open” is displayed on screen. 3 — 40 Perfusor® Space, 1.2 gb… -
Page 95
Disassembly / Assembly 10. Select the desired file with the mouse pointer and press the “Open” button. The data loaded is displayed on screen. 11. Actuate the “To device” button. Fig.: 3 — 51 Fig.: 3 — 52 Perfusor® Space, 1.2 gb 3 — 41… -
Page 96
Disassembly / Assembly 12. Press the “OK” button when the window “Confirmation” is displayed. Fig.: 3 — 53 13. Enter your worker id in the window “Worker ID”. Press the “OK” button to transmit the data to the device. Fig.: 3 — 54 A message is displayed that a change of the device data may affect the patients. -
Page 97
Disassembly / Assembly Loudspeaker 1. Route the loudspeaker connection cable between the drive motor and its connection wires, see Fig.: 3 — Fig.: 3 — 56 Piston Brake WARNING PAY ATTENTION TO THE PISTON BRAKE BLADE WHEN WORKING ON THE PISTON BRAKE. THE BLADE IS SHARP AND MAY CAUSE INJURIES. -
Page 98
Disassembly / Assembly Drive Head 1. Insert the drive head PCB with the strain gauge vertically in the drive head cover. Please note that the strain gauge must not get jammed in the guide. Note The three strain gauge tongues are level with the PCB upper side. 2. -
Page 99
Disassembly / Assembly Note Check the position of the emergency release tappet before mounting the drive head housing. Fig.: 3 — 60 5. Push the drive head housing on to the driving tube until the switch flag of the membrane tappet is positioned just before the claw mechanism. -
Page 100
Disassembly / Assembly Note Pay attention to the syringe wing sensor when inserting the side part of the housing and make sure that the sensor can be operated correctly. 1. Route the ribbon cable through the slide nut before pushing in the driving tube and then through the slide in front of the spindle nut when looking from the rear of the housing. -
Page 101
Disassembly / Assembly Operating Unit Note Before fitting the rear panel check the screw lengths. The screws may only be 4.5 mm long (without head). Longer screws must be exchanged. Note Before mounting the operating unit check the plastic foam gaskets in the housing opening for the connection cable to the operating unit. -
Page 102
Disassembly / Assembly Check List for Checks after Repair Visual Inspection Electrical Safety Functional Inspection according to IEC/EN 60601-1 or VDE 0750 and VDE 0751 Cleanliness The patient and housing leakage current of Locking with second unit Completeness the Perfusor® Space is caused exclusively by Operating unit magnets the operating voltage supply (Power Supply Battery compartment cover… -
Page 103
Disassembly / Assembly Visual Inspection Electrical Safety Functional Inspection according to IEC/EN 60601-1 or VDE 0750 and VDE 0751 Pressure cut off according to TSC Syringe type: «#Lehre OPS50“ Delivery rate: 200 ml/h WARNING REMOVE SYRINGE GAUGE ONLY WHEN RELEASED. DANGER OF INJURY! Strain gauge pressure measurement Pressure stage 1 _______ N… -
Page 104
Disassembly / Assembly For your notes: 3 — 50 Perfusor® Space, 1.0 gb… -
Page 105
For disinfection by wiping, you should use for example Meliseptol® from B. Braun. Allow the unit to dry for at least one minute. When you disinfect the device by spraying, make sure not to spray in the system openings (such as interface sockets and connectors, loudspeaker opening). -
Page 106
Servicing the Unit For your notes: 4 — 2 Perfusor® Space, 1.0 gb… -
Page 107
Checklist for Technical Safety Check – Every 24 Months Unit: Perfusor® Space User Manufacturer: B. Braun Melsungen AG Observe the Service Manual and the instructions for use. All measured values are to be documented. Accessories used should be included in testing. Make exclusive use of calibrated measuring equipment. -
Page 108
Technical Safety Check (TSC) Index d (Master — to be added to the documentation) Visual Inspection Electrical Safety Functional Inspection according to IEC/EN 60601-1 or VDE 0750 and VDE 0751 Pressure cut-off Syringe type: «#Lehre OPS50“ Delivery rate: 200 ml/h — Strain gauge pressure measurement Pressure stage 1 (9 … -
Page 109
Checklist for Technical Safety Check – Every 24 Months Unit: Power Supply SP User Manufacturer: B. Braun Melsungen AG Observe the Service Manual and the instructions for use of the respective device. All measured values are to be documented. Make exclusive use of calibrated measuring equipment. -
Page 110
Technical Safety Check (TSC) Index d (Master — to be added to the documentation) For your notes: M001 32 10 05 F04 / 3891 6142 (GB) 6 — 2 Perfusor® Space, 1.0 gb Sheet 2 of 2… -
Page 111
Procedural Instructions on the TSC Visual Inspection Perfusor® Space 1. Check the Perfusor® Space and accessories for cleanliness. 2. Check the Perfusor® Space and accessories for completeness and check configuration. 3. Check the Perfusor® Space and its accessories for damage and the labels for readability. -
Page 112
Procedural Instructions on the TSC Electrical Safety Perfusor® Space according to IEC/EN 60601-1 The patient and housing leakage current of the Perfusor® Space is caused exclusively by the operating voltage supply (Power Supply or VDE 0750 and VDE 0751 SP or SpaceStation). The Technical Safety Checks of the power supply SP (drawing No. -
Page 113
Procedural Instructions on the TSC Functional Inspection Perfusor® Space Mechanical Inspection 1. Fit the unit to be tested on top of another Space device and check the proper functioning of the lock. 2. Fit the unit to be tested under another Space device and check the proper functioning of the lock. -
Page 114
Procedural Instructions on the TSC 3. Check syringe recognition. a) Insert approved 2 ml / 3 ml syringe. The syringe size is recognized. b) Insert approved 50 ml / 3 ml syringe. The syringe size is recognized. 4. Carry out infusion and bolus with any syringe and press all buttons at least once. -
Page 115
Procedural Instructions on the TSC Pressure Cut-Off (Strain Gauge Pressure Measurement) WARNING DURING THE STRAIN GAUGE MEASUREMENT WITH SYRINGE GAUGE THE SYRINGE HOLDER MUST NOT BE OPENED. THE SYRINGE GAUGE IS UNDER VERY HIGH PRESSURE AND MAY CAUSE INJURIES IF THE PRESSURE IS RELIEVED SUDDENLY. 1. -
Page 116
Procedural Instructions on the TSC 3. Disassemble push-button plate for Perfusor® Space from syringe gauge, insert syringe gauge and select syringe type “#Lehre OPS50”. Note The threaded end of the syringe gauge must be introduced in the opening of the motor power test adapter. Hold on to syringe gauge –… -
Page 117
Test Equipment and Special Tools Test equipment Designation Ord. No. For Device Check Syringe 2 ml / 3 ml Syringe 10 ml Syringe 30 ml Service connector SP ……3452 1062 HiBaSeD Service-CD . -
Page 118
Test Equipment and Special Tools Syringe gauge PSP ……0770 5204 “#Lehre OPS 50” with push-button plate and motor power test adapter for Perfusor®… -
Page 119
Test Equipment and Special Tools Special Tools Designation Ord. No. For Repairs TORX screwdriver kit 5 — 10, 25 TORX plus screwdriver kit 5 — 10, 25 Screwdriver 6IPx60 TORX plus ….4002 4806 Screwdriver 8IPx60 TORX plus . -
Page 120
Test Equipment and Special Tools For your notes: 8 — 4 Perfusor® Space, 1.0 gb… -
Page 121
Spare Parts List Item Designation Ord. No. Perfusor® Space Service part kit Perfusor® Space … . . 3477 4270 with: housing cover cap (40 pieces) cover caps for operating unit (10 pieces) cover cap for syringe holder (10 pieces) cover cap for drive head and claw (20 pieces) housing foot (20 pieces) -
Page 122
Spare Parts List Fig.: 9 — 1 Exploded drawing Perfusor® Space 9 — 2 Perfusor® Space, 1.3 gb… -
Page 123
Spare Parts List Battery compartment cover PSP , cpl..3452 0872 Battery pack SP (NIMH) ….0871 3180 Operating unit PSP, cpl. -
Page 124
Spare Parts List 9 — 4 Perfusor® Space, 1.0 gb… -
Page 125
Revision Documentation 10 — Description of Version Version 1.0 (Base Version) First edition of this Service Manual Release date: 07.01.05. Version 1.1 Description for disassembly of the drive head added. Claw mechanism and drive head PCB added as new spare parts. -
Page 126
Revision Documentation 10 — 2 Perfusor® Space, 1.0 gb… -
Page 127
Revision Documentation Title page and pages preceding main body Page 2 — 9 ……..Version 1.1 Page 0 — 1 . -
Page 128
Revision Documentation Page 3 — 13 ……..Version 1.0 Page 3 — 49 . -
Page 129
Revision Documentation Page 10 — 3 ……..Version 1.0 Page 10 — 4 . -
Page 130
Revision Documentation 10 — 6 Perfusor® Space, 1.0 gb… -
Page 131
Index 11 — Accessories ……..1 — 12 Housing Alarms .
B.Braun Space – насос инфузионный шприцевый (Перфузор)
Многофункциональный инфузионный шприцевый насос B.Braun Space (Перфузор) используется для внутривенного введения лекарственных препаратов пациентам, которые нуждаються в постоянной инфузионной терапии, а также для обеспечения энтерального питания. Аппарат незаменим в анестезиологии, реаниматологии, хирургии.
Насос подходит как для постоянной, так и для периодической, для энтеральной либо парентеральной подачи жидкости, когда лекарство не попадает в желудочно-кишечный тракт. Компактный и легкий, прибор легко подключается к коммуникационным сетям.
Одной из отличительных характеристик данной модели является безопасность инфузии. Во избежание ошибок в определении количества подаваемых препаратов расчет дозы происходит автоматически. При этом устройство учитывает время терапии, объем лекарства, его концентрацию.
В случае возникновения безопасности срабатывает световой индикатор. А при внезапном повышении давления звучит сигнал тревоги и скорость болюса уменьшается автоматически. Инфузия прекращается, если системой обнрауживается неточность дозировки от 0,1 мл.
Преимущества инфузионного насоса
Модульная система B.Braun Space отличается простотой управления и эргономичностью. Доступный каждому интерфейс, удобное меню и интуитивно понятная навигация позволяют без специального обучения задавать показатели и менять параметры, контролируя состояние пациента.
Стоит выделить другие преимущества насоса:
-
Библиотека препаратов на 30 категорий, которые включают до 1 500 позиций. Для каждого лекарства предусмотрены ключевые данные и рекомендованные настройки инфузии.
-
Есть готовые заданные параметры и возможность установить ограничения исходя из целей и специфики инфузии.
-
Свободный поток питательной смеси или лекарства обеспечивается наличием интегрированного фиксатора шприцевого штока.
-
Менять шприц удобно и безопасно за счет автоматического привода, который также улучшает пусковые свойства.
Возможность работы от перезаряжаемого аккумулятора NiMH составляет восемь часов (при 25 мл/ч). С учетом малого веса (1,4 кг) и небольших габаритов, насос является удобным устройством для автономного проведения инфузии.
Шаг установки скорости:
-
0.01* — 99.99 мл/ч, шаг 0.01 мл/ч;
-
100.0 — 999.9 мл/ч, шаг 0.1 мл/ч;
-
точность болюсной инфузии: тип ±2%;
-
макс. болюс, после снижения скорости: ≤ 0.2 мл.
Скорость инфузии в режиме KVO:
-
Скорость ≥ 10 мл/ч: KVO 3 мл/ч;
-
Скорость < 10 мл/ч: KVO 1 мл/ч;
-
Скорость < 1 мл/ч: KVO = заданной скорости.
Установка объема инфузии:
-
0,1 — 99,99 мл, шаг 0,01 мл;
-
100,0 — 999,9 мл, шаг 0,1 мл;
-
1000 — 9999 мл, шаг 1 мл.
Протокол событий:
-
1000 последних вводов данных;
-
100 событий диагностики системы;
-
Источник питания: 100-240В АВ, 50/60 Гц, 11-16В DC (от Станции Спэйс или опционального блока питания);
-
Вызов персонала макс. 24В / 0,5А / 24 ВА (VDE 0834).
Условия эксплуатации:
-
Относительная влажность: 30% … 90% (без конденсата);
-
Температура: +5… +40° С;
-
Атмосферное давление: 500… 1060 мбар.
|
Скорость длительной инфузии / скорость болюса зависит от типа шприца |
||
|
Размер шприца, мл
|
Скорость болюса, мл/ч |
|
|
50/60
|
Скорость инфузии, мл/ч 0.01 — 200, опционально 0.01 — 999.9 |
1 — 1800 |
|
30/35
|
0.01 — 100 |
1 — 1200 |
|
20 |
0.01 — 100 |
1 — 800 |
|
10/12 |
0.01 — 50 |
1 — 500 |
|
5/6 |
0.01 — 50 |
1 — 300 |
|
2/3 |
0.01 — 25 |
1 — 150 |
Свяжитесь со специалистами магазина «Бравокислород» по телефону 8 (343) 357-90-06 или 8 (800) 775-16-38. Вы получите бесплатную консультацию и помощь с подбором инфузионного оборудования для дома или для медицинского учреждения. Работаем с государственными и частными клиниками по договору согласно ФЗ 44 и 223.
Гарантия: 1 год

