From Wikipedia, the free encyclopedia
 |
|
| Clinical data | |
|---|---|
| Trade names | Drolban, Masteril, Masteron, others |
| Other names | Dromostanolone propionate; NSC-12198; Drostanolone 17β-propionate; 2α-Methyl-4,5α-dihydrotestosterone 17β-propionate; 2α-Methyl-DHT propionate; 2α-Methyl-5α-androstan-17β-ol-3-one 17β-propionate |
| Pregnancy category |
|
| Routes of administration |
Intramuscular injection[1] |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 0–2% Intramuscular: 100% |
| Protein binding | High |
| Metabolism | Hepatic |
| Elimination half-life | Intramuscular: 2 days[1] |
| Excretion | Urine |
| Identifiers | |
|
IUPAC name
|
|
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| ChEBI |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.007.550 |
| Chemical and physical data | |
| Formula | C23H36O3 |
| Molar mass | 360.538 g·mol−1 |
| 3D model (JSmol) |
|
|
SMILES
|
|
|
InChI
|
|
| (verify) |
Drostanolone propionate, or dromostanolone propionate, sold under the brand names Drolban, Masteril, and Masteron among others, is an androgen and anabolic steroid (AAS) medication which was used to treat breast cancer in women but is now no longer marketed.[1][2] It is given by injection into muscle.[1]
Side effects of drostanolone propionate include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[1] It has no risk of liver damage.[1] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[1][3] It has moderate anabolic effects and weak androgenic effects, which give it a mild side effect profile and make it especially suitable for use in women.[1] The drug has no estrogenic effects.[1] Drostanolone propionate is an androgen ester and a long-lasting prodrug of drostanolone in the body.[1]
Drostanolone propionate was first described in 1959 and was introduced for medical use in 1961.[1][4][5] In addition to its medical use, drostanolone propionate is used to improve physique and performance.[1] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[1][6]
Medical uses[edit]
The principal clinical indication of drostanolone propionate in the United States as well as international markets was the treatment of advanced inoperable breast cancer in women.[1]
Hormonal treatment is part of the complex therapy for some kind of tumors, particularly the ones associated with hormone-active tissues like breast or prostate cancer. Some types of breast cancer cells, expressing estrogen receptors (called ER+ cancers), use estrogen for their growth and dissemination. That is why drugs that block estrogen receptors or decrease their expression on the cell membrane, antiestrogens, could limit the tumor spread and size. Drostanolone propionate has been FDA approved[7] as an antiestrogenic drug for the treatment of breast cancer. By the time of its release, there were not many alternatives for patients with breast cancer and drostanolone propionate was a revolution for these patients. As it has lower androgenic rate compared to testosterone, the risk of virilization is much lighter. Due to this fact, women, who usually do not respond well to any AAS, were having much greater chance to survive cancer. Drostanolone propionate can also be used for breast tumors that do not respond well to other treatments or also as palliative care for advanced incurable tumors. The effects of the product depend of course on the dose and period of administration. The risk of virilization becomes greater with high doses and continuous administration period.
| Route | Medication | Form | Dosage |
|---|---|---|---|
| Oral | Methyltestosterone | Tablet | 30–200 mg/day |
| Fluoxymesterone | Tablet | 10–40 mg 3x/day | |
| Calusterone | Tablet | 40–80 mg 4x/day | |
| Normethandrone | Tablet | 40 mg/day | |
| Buccal | Methyltestosterone | Tablet | 25–100 mg/day |
| Injection (IMTooltip intramuscular injection or SCTooltip subcutaneous injection) | Testosterone propionate | Oil solution | 50–100 mg 3x/week |
| Testosterone enanthate | Oil solution | 200–400 mg 1x/2–4 weeks | |
| Testosterone cypionate | Oil solution | 200–400 mg 1x/2–4 weeks | |
| Mixed testosterone esters | Oil solution | 250 mg 1x/week | |
| Methandriol | Aqueous suspension | 100 mg 3x/week | |
| Androstanolone (DHT) | Aqueous suspension | 300 mg 3x/week | |
| Drostanolone propionate | Oil solution | 100 mg 1–3x/week | |
| Metenolone enanthate | Oil solution | 400 mg 3x/week | |
| Nandrolone decanoate | Oil solution | 50–100 mg 1x/1–3 weeks | |
| Nandrolone phenylpropionate | Oil solution | 50–100 mg/week | |
| Note: Dosages are not necessarily equivalent. Sources: See template. |
Non-medical uses[edit]
Drostanolone propionate is or has been used for physique- and performance-enhancing purposes by competitive athletes, bodybuilders, and powerlifters.[1]
Side effects[edit]
Drostanolone propionate produces considerably less virilization in women compared to equal doses of testosterone propionate.[1] However, since the given dosage for breast cancer was relatively high (200 mg/twice a week),[8] mild virilization including oily skin, acne, voice deepening, hirsutism, and clitoral enlargement could still occur, and marked virilization could manifest with long-term therapy.[1] The drug has no estrogenic activity and hence has no propensity for causing gynecomastia (in males) or fluid retention.[1] Drostanolone propionate is not known to pose a risk of hepatotoxicity.[9][1]
Pharmacology[edit]
Pharmacodynamics[edit]
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. |
Drostanolone propionate is a prodrug of drostanolone.[1] Like other AAS, drostanolone is an agonist of the androgen receptor (AR).[1] It is not a substrate for 5α-reductase and is a poor substrate for 3α-hydroxysteroid dehydrogenase (3α-HSD), and therefore shows a high ratio of anabolic to androgenic activity.[1] As a DHT derivative, drostanolone is not a substrate for aromatase and hence cannot be aromatized into estrogenic metabolites.[1] While no data are available on the progestogenic activity of drostanolone, it is thought to have low or no such activity similarly to other DHT derivatives.[1] Since the drug is not 17α-alkylated, it is not known to cause hepatotoxicity.[1]
Drostanolone propionate, via its active form drostanolone, interacts with the AR and activates a cascade of genetic changes, including increased protein synthesis (anabolism) and decreased amino acid degradation (catabolism). It also induces a reduction or inhibition of prolactin or estrogen receptors in the breasts, which is linked to its antitumor effects.[10]
Pharmacokinetics[edit]
Drostanolone propionate is not active via the oral route and must be administered via intramuscular injection.[1] The elimination half-life of the drug via this route is approximately 2 days.[1] It has a much longer elimination half-life via intramuscular injection than drostanolone.[1] Drostanolone propionate is metabolized into drostanolone, which is the active form.[1]
Chemistry[edit]
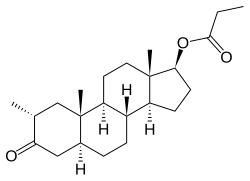
Drostanolone propionate, or drostanolone 17β-propionate, is a synthetic androstane steroid and a derivative of DHT.[11][12][1] It is the C17β propionate (propanoate) ester of drostanolone, which itself is 2α-methyl-4,5α-dihydrotestosterone (2α-methyl-DHT) or 2α-methyl-5α-androstan-17β-ol-3-one.[11][12][1]
| Anabolic steroid | Structure | Ester | Relative mol. weight |
Relative AAS contentb |
Durationc | |||
|---|---|---|---|---|---|---|---|---|
| Position | Moiety | Type | Lengtha | |||||
| Boldenone undecylenate | C17β | Undecylenic acid | Straight-chain fatty acid | 11 | 1.58 | 0.63 | Long | |
| Drostanolone propionate | C17β | Propanoic acid | Straight-chain fatty acid | 3 | 1.18 | 0.84 | Short | |
| Metenolone acetate | C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.14 | 0.88 | Short | |
| Metenolone enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.37 | 0.73 | Long | |
| Nandrolone decanoate | C17β | Decanoic acid | Straight-chain fatty acid | 10 | 1.56 | 0.64 | Long | |
| Nandrolone phenylpropionate | C17β | Phenylpropanoic acid | Aromatic fatty acid | – (~6–7) | 1.48 | 0.67 | Long | |
| Trenbolone acetate | C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.16 | 0.87 | Short | |
| Trenbolone enanthated | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | Long | |
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative androgen/anabolic steroid content by weight (i.e., relative androgenic/anabolic potency). c = Duration by intramuscular or subcutaneous injection in oil solution. d = Never marketed. Sources: See individual articles. |
History[edit]
Drostanolone and drostanolone propionate were first described in 1959.[1][4] The related AAS oxymetholone and methasterone (methyldrostanolone) were first described in the same paper as well.[1] Drostanolone propionate was introduced for medical use in the United States in 1961 and in Europe shortly thereafter.[5]
Society and culture[edit]
Generic names[edit]
Drostanolone propionate is the generic name of the drug and its BANMTooltip British Approved Name, while dromostanolone propionate is the USANTooltip United States Adopted Name and USPTooltip United States Pharmacopeia; there is no INNTooltip International Nonproprietary Name for this form.[11][12][13] The generic name of the unesterified form of the drug is drostanolone or dromostanolone and the former is its INNTooltip International Nonproprietary Name, BANTooltip British Approved Name, and DCFTooltip Dénomination Commune Française while there is no USANTooltip United States Adopted Name.[11][12][13][2]
Brand names[edit]
Drostanolone propionate was marketed under a variety of brand names including Drolban, Masterid, Masteril, Masteron, Masterone, Mastisol, Metormon, Permastril, and Prometholone.[11][12][1]
Availability[edit]
Drostanolone propionate appears to no longer be marketed.[1][2] It was previously available in the United States, Europe, and Japan.[12][1] In Europe, it was specifically marketed in the United Kingdom, Germany, Belgium, France, Spain, Portugal, Italy, and Bulgaria.[12][1]
Legal status[edit]
Drostanolone propionate, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act.[6]
References[edit]
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 517–. ISBN 978-0-9828280-1-4.
- ^ a b c «Anabolic Agents». Drugs.com.
- ^ Kicman AT (June 2008). «Pharmacology of anabolic steroids». British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ^ a b Ringold HJ, Batres E, Halpern O, Necoechea E (1959). «Steroids. CV.12-Methyl and 2-Hydroxymethylene-androstane Derivatives». Journal of the American Chemical Society. 81 (2): 427–432. doi:10.1021/ja01511a040. ISSN 0002-7863.
- ^ a b William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1402–. ISBN 978-0-8155-1856-3.
- ^ a b Karch SB (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
- ^ «Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations». www.accessdata.fda.gov. Retrieved 2016-03-15.
- ^ «Drostanolone propionate (Masteron) administration — The Dose for Treating Breast Cancer». Masterone. 2020-01-24. Archived from the original on 2020-10-01. Retrieved 2021-02-23.
- ^ Solimini R, Rotolo MC, Mastrobattista L, Mortali C, Minutillo A, Pichini S, et al. (March 2017). «Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping». European Review for Medical and Pharmacological Sciences. 21 (1 Suppl): 7–16. PMID 28379599.
- ^ «Drostanolone (PIM 901)». www.inchem.org. Retrieved 2016-03-15.
- ^ a b c d e Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 652–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f g Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 377–. ISBN 978-3-88763-075-1.
- ^ a b Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 106–. ISBN 978-94-011-4439-1.
External links[edit]
Rec.INN
зарегистрированное ВОЗ
Фармакологическое действие
Андрогенное средство. Оказывает противоопухолевое действие при раке молочной железы с метастазами.
Показания активного вещества
ДРОСТАНОЛОН
Рак молочной железы с метастазами.
Режим дозирования
Устанавливают индивидуально, в зависимости от показаний, стадии заболевания, схемы противоопухолевой терапии.
Побочное действие
Возможно: токсический гепатит, гиперкальциемия, гиперкальциурия, вирилизация.
Противопоказания к применению
Острые заболевания печени и почек, гиперкальциемия, сердечно-сосудистые заболевания, терминальная стадия заболевания.
Применение при нарушениях функции печени
Противопоказан при острых заболеваниях печени.
Применение при нарушениях функции почек
Противопоказан при острых заболеваниях почек.
Особые указания
Применяют у женщин с сохраненным менструальным циклом или при менопаузе до 5 лет. Более эффективен после двусторонней овариэктомии или подавления функции яичников в результате облучения.
Химическое название
2a-methyl-dihydro-testosterone propionate
Химические свойства
Анаболик, стероид, выпускается в виде дростанолона пропионата и дростанолона энантата. Обладает достаточно высокой андрогенной активностью и умеренным анаболическим действием. Впервые это вещество было описано в 1959 году, в продажу поступило в 70-х годах. Белый, мелкокристаллический порошок.
Дростанолон, как правило, выпускают в инъекционной форме в виде раствора, дозировкой по 50 или 100 мг на мл.
Фармакологическое действие
Анаболическое, андрогенное.
Фармакодинамика и фармакокинетика
Дростанолон снижает выработку фолликулостимулирующего и лютеотропного гормонов. Андрогенный эффект лекарства выражен меньше, чем у тестостерона, а анаболический – больше. Также средство обладает противоопухолевой активностью, даже на стадиях с метастазами. Стимулирует процессы синтеза белковых молекул, ингибирует процессы отложения жира, ускоряется процесс фиксации кальция в костях и нарастание мышечной массы. Стимулирует выработку эритропоэтина.
Дростанолона пропионат проникая в кровеносную систему, распадается на свободное активное вещество и эфир. Таким образом увеличивается время, в течение которого проявляется терапевтический эффект от приема лекарства. Период полувыведения составляет порядка двух суток.
Показания к применению
Применяется для лечения рака молочной железы.
Противопоказания
Противопоказан к приему:
- при острых заболеваниях почек и печени;
- пациентам с гиперкальциемией;
- больным сердечно-сосудистыми заболеваниями;
- в терминальной стадии рака.
Побочные действия
Дростанолон может вызвать следующие побочные реакции:
- акне, алопеция, агрессивное поведение, гиперкальциурия;
- гипертрофия простаты, токсический гепатит;
- вирилизация, гиперкальциемия.
Инструкция по применению (Способ и дозировка)
Дозировка Дростанолона определяется в индивидуальном порядке.
Для лечения рака молочной железы врачи назначают разные схемы лечения, в зависимости от стадии, площади новообразования и сопутствующих заболеваний.
Спортсмены обычно принимают от 300 до 500 мг средства за 7 дней, кратность инъекций – каждый третий день или через день. Курс лечения от 6 до 12 недель.
Передозировка
Нет сведений о передозировке препаратом.
Взаимодействие
При применении Дростанолона спортсменами, его часто сочетают с нандролоном, болденона ундесилинатом, метандростанолоном, тестостероном, станозололом, тренболоном, и так далее.
Условия продажи
На данный момент приобрести лекарство в аптеке невозможно.
Особые указания
Для лечения рака молочной железы средство используют для женщин, у которых сохранился менструальный цикл или менопауза длится менее 5 лет.
Наибольшей эффективности лечения можно достигнуть после двусторонней овариэктомии или облучения для подавления функции яичников.
Препараты, в которых содержится (Аналоги)
Совпадения по коду АТХ 4-го уровня:
Торговое название средства: Медротестрон (в масляном растворе), Метормон, Дролбан, Мастерон.
Отзывы
Отзывов о применении лекарства для лечения рака практически нет, так как на данный моменты его не используют. В основном встречаются сообщения о применении препаратов Дростанолона спортсменами. Хорошие отзывы на вещество, когда его используют в качестве жиросжигателя в сочетании с другими средствами. Стероид не рекомендуют женщинам.
Цена, где купить
На данный момент нельзя указать актуальную стоимость лекарства, так как оно отсутствует в свободной продаже в аптеках.
Дростанолон
Drostanolone
Фармакологическое действие
Дростанолон — анаболический андрогенный стероид (ААС), оказывает противоопухолевое действие при раке молочной железы с метастазами.
Фармакодинамика
Обладает андрогенным (выражено слабее по сравнению с тестостерона пропионатом) и анаболическим (более выражено, чем у тестостерона пропионата) эффектами. Оказывает противоопухолевое действие при раке вилочковой железы, в том числе при наличии метастазов, а также у больных, у которых функция яичников подавлена облучением, андрогенными препаратами или подвергшихся овариэктомии. Более эффективен при двусторонней овариоэктомии.
Фармакокинетика
Период полувыведения (T½) составляет около 48 часов.
Показания
Рак молочной железы с метастазами.
Противопоказания
- Повышенная чувствительность к дростанолону;
- острые заболевания печени, почек, сердечно-сосудистой системы (инфаркт миокарда, тяжёлая форма артериальной гипертензии, гипертонический криз);
- гиперкальциемия;
- терминальная стадия заболевания;
- беременность или кормление грудью.
Беременность и грудное вскармливание
Применение при беременности
Категория действия на плод по FDA — N.
Адекватных и строго контролируемых исследований о возможности применения дростанолона у беременных женщин не проведено.
Применение андрогенов у беременных женщин, особенно в течение Ⅰ триместра беременности, вызывает вирилизацию наружных половых органов женского плода. Эта вирилизация включает клиторомегалию, аномальное развитие влагалища и слияние генитальных складок с образованием мошоночноподобной структуры. Степень маскулинизации зависит от количества препарата и возраста плода. Чаще всего, это происходит у плода женского пола, когда препараты вводятся в Ⅰ триместре.
При использовании больших доз андрогенов может возникнуть олигоспермия.
Женщины репродуктивного возраста и мужчины, ведущие половую жизнь, во время применения должны использовать надёжные методы контрацепции. В случае наступления беременности, отсутствия менструации или при подозрении на возможную беременность пациентка должна сообщить об этом своему лечащему врачу. При обнаружении беременности применение дростанолона следует прекратить. Если дростанолон применяется в период беременности или беременность наступила во время лечения, пациентка должна быть предупреждена о потенциальной опасности для плода.
Применение дростанолона у беременных женщин противопоказано.
Применение в период грудного вскармливания
Специальных исследований по безопасности применения дростанолона в период грудного вскармливания не проведено.
Неизвестно, выделяется ли дростанолон в человеческое грудное молоко. Риск для грудного ребёнка не может быть исключён.
Рекомендуется прекратить грудное вскармливание в случае применения дростанолона.
Способ применения и дозы
Режим дозирования индивидуальный, в зависимости от показаний, стадии заболевания, схемы противоопухолевой терапии и лекарственной формы.
Побочные действия
Токсический гепатит, гиперкальциемия, гиперкальциурия, нарушение сердечной деятельности, вирилизация.
Особые указания
При развитии побочных явлений введение дростанолона следует временно приостановить.
Применяют у женщин с сохранённом менструальным циклом или при менопаузе до 5 лет.
Более эффективен после двусторонней овариэктомии или подавления функции яичников в результате облучения.
Спортивная медицина
Дростанолон может стать причиной нарушений антидопинговых правил и положительного допинг теста.
Дростанолон включён в список анаболических андрогенных стероидов (ААС) запрещённых WADA.
Глоссарий
| Термин | Описание |
|---|---|
| WADA | Всемирное антидопинговое агентство |
Классификация
-
АТХ
L01
-
Фармакологические группы
-
Код МКБ 10
-
Категория при беременности по FDA
N
(не классифицировано FDA)
Информация о действующем веществе Дростанолон предназначена для медицинских и фармацевтических специалистов, исключительно в справочных целях. Инструкция не предназначена для замены профессиональной медицинской консультации, диагностики или лечения. Содержащаяся здесь информация может меняться с течением времени. Наиболее точные сведения о применении препаратов, содержащих активное вещество Дростанолон, содержатся в инструкции производителя, прилагаемой к упаковке.
Drostanolone propionate, or dromostanolone propionate, sold under the brand names Drolban, Masteril, and Masteron among others, is an androgen and anabolic steroid (AAS) medication which was used to treat breast cancer in women but is now no longer marketed.[1][2] It is given by injection into muscle.[1]
 |
|
| Clinical data | |
|---|---|
| Trade names | Drolban, Masteril, Masteron, others |
| Other names | Dromostanolone propionate; NSC-12198; Drostanolone 17β-propionate; 2α-Methyl-4,5α-dihydrotestosterone 17β-propionate; 2α-Methyl-DHT propionate; 2α-Methyl-5α-androstan-17β-ol-3-one 17β-propionate |
| Pregnancy category |
|
| Routes of administration |
Intramuscular injection[1] |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 0–2% Intramuscular: 100% |
| Protein binding | High |
| Metabolism | Hepatic |
| Elimination half-life | Intramuscular: 2 days[1] |
| Excretion | Urine |
| Identifiers | |
|
IUPAC name
|
|
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| ChEBI |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.007.550 |
| Chemical and physical data | |
| Formula | C23H36O3 |
| Molar mass | 360.538 g·mol−1 |
| 3D model (JSmol) |
|
|
SMILES
|
|
|
InChI
|
|
| (verify) |
Side effects of drostanolone propionate include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[1] It has no risk of liver damage.[1] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[1][3] It has moderate anabolic effects and weak androgenic effects, which give it a mild side effect profile and make it especially suitable for use in women.[1] The drug has no estrogenic effects.[1] Drostanolone propionate is an androgen ester and a long-lasting prodrug of drostanolone in the body.[1]
Drostanolone propionate was first described in 1959 and was introduced for medical use in 1961.[1][4][5] In addition to its medical use, drostanolone propionate is used to improve physique and performance.[1] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[1][6]
Medical uses
Edit
The principal clinical indication of drostanolone propionate in the United States as well as international markets was the treatment of advanced inoperable breast cancer in women.[1]
Hormonal treatment is part of the complex therapy for some kind of tumors, particularly the ones associated with hormone-active tissues like breast or prostate cancer. Some types of breast cancer cells, expressing estrogen receptors (called ER+ cancers), use estrogen for their growth and dissemination. That is why drugs that block estrogen receptors or decrease their expression on the cell membrane, antiestrogens, could limit the tumor spread and size. Drostanolone propionate has been FDA approved[7] as an antiestrogenic drug for the treatment of breast cancer. By the time of its release, there were not many alternatives for patients with breast cancer and drostanolone propionate was a revolution for these patients. As it has lower androgenic rate compared to testosterone, the risk of virilization is much lighter. Due to this fact, women, who usually do not respond well to any AAS, were having much greater chance to survive cancer. Drostanolone propionate can also be used for breast tumors that do not respond well to other treatments or also as palliative care for advanced incurable tumors. The effects of the product depend of course on the dose and period of administration. The risk of virilization becomes greater with high doses and continuous administration period.
| Route | Medication | Form | Dosage |
|---|---|---|---|
| Oral | Methyltestosterone | Tablet | 30–200 mg/day |
| Fluoxymesterone | Tablet | 10–40 mg 3x/day | |
| Calusterone | Tablet | 40–80 mg 4x/day | |
| Normethandrone | Tablet | 40 mg/day | |
| Buccal | Methyltestosterone | Tablet | 25–100 mg/day |
| Injection (IMTooltip intramuscular injection or SCTooltip subcutaneous injection) | Testosterone propionate | Oil solution | 50–100 mg 3x/week |
| Testosterone enanthate | Oil solution | 200–400 mg 1x/2–4 weeks | |
| Testosterone cypionate | Oil solution | 200–400 mg 1x/2–4 weeks | |
| Mixed testosterone esters | Oil solution | 250 mg 1x/week | |
| Methandriol | Aqueous suspension | 100 mg 3x/week | |
| Androstanolone (DHT) | Aqueous suspension | 300 mg 3x/week | |
| Drostanolone propionate | Oil solution | 100 mg 1–3x/week | |
| Metenolone enanthate | Oil solution | 400 mg 3x/week | |
| Nandrolone decanoate | Oil solution | 50–100 mg 1x/1–3 weeks | |
| Nandrolone phenylpropionate | Oil solution | 50–100 mg/week | |
| Note: Dosages are not necessarily equivalent. Sources: See template. |
Non-medical uses
Edit
Drostanolone propionate is or has been used for physique- and performance-enhancing purposes by competitive athletes, bodybuilders, and powerlifters.[1]
Side effects
Edit
Drostanolone propionate produces considerably less virilization in women compared to equal doses of testosterone propionate.[1] However, since the given dosage for breast cancer was relatively high (200 mg/twice a week),[8] mild virilization including oily skin, acne, voice deepening, hirsutism, and clitoral enlargement could still occur, and marked virilization could manifest with long-term therapy.[1] The drug has no estrogenic activity and hence has no propensity for causing gynecomastia (in males) or fluid retention.[1] Drostanolone propionate is not known to pose a risk of hepatotoxicity.[9][1]
Pharmacology
Edit
Pharmacodynamics
Edit
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. |
Drostanolone propionate is a prodrug of drostanolone.[1] Like other AAS, drostanolone is an agonist of the androgen receptor (AR).[1] It is not a substrate for 5α-reductase and is a poor substrate for 3α-hydroxysteroid dehydrogenase (3α-HSD), and therefore shows a high ratio of anabolic to androgenic activity.[1] As a DHT derivative, drostanolone is not a substrate for aromatase and hence cannot be aromatized into estrogenic metabolites.[1] While no data are available on the progestogenic activity of drostanolone, it is thought to have low or no such activity similarly to other DHT derivatives.[1] Since the drug is not 17α-alkylated, it is not known to cause hepatotoxicity.[1]
Drostanolone propionate, via its active form drostanolone, interacts with the AR and activates a cascade of genetic changes, including increased protein synthesis (anabolism) and decreased amino acid degradation (catabolism). It also induces a reduction or inhibition of prolactin or estrogen receptors in the breasts, which is linked to its antitumor effects.[10]
Pharmacokinetics
Edit
Drostanolone propionate is not active via the oral route and must be administered via intramuscular injection.[1] The elimination half-life of the drug via this route is approximately 2 days.[1] It has a much longer elimination half-life via intramuscular injection than drostanolone.[1] Drostanolone propionate is metabolized into drostanolone, which is the active form.[1]
Chemistry
Edit
Drostanolone propionate, or drostanolone 17β-propionate, is a synthetic androstane steroid and a derivative of DHT.[11][12][1] It is the C17β propionate (propanoate) ester of drostanolone, which itself is 2α-methyl-4,5α-dihydrotestosterone (2α-methyl-DHT) or 2α-methyl-5α-androstan-17β-ol-3-one.[11][12][1]
| Anabolic steroid | Structure | Ester | Relative mol. weight |
Relative AAS contentb |
Durationc | |||
|---|---|---|---|---|---|---|---|---|
| Position | Moiety | Type | Lengtha | |||||
| Boldenone undecylenate | |
C17β | Undecylenic acid | Straight-chain fatty acid | 11 | 1.58 | 0.63 | Long |
| Drostanolone propionate | |
C17β | Propanoic acid | Straight-chain fatty acid | 3 | 1.18 | 0.84 | Short |
| Metenolone acetate | |
C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.14 | 0.88 | Short |
| Metenolone enanthate | |
C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.37 | 0.73 | Long |
| Nandrolone decanoate | |
C17β | Decanoic acid | Straight-chain fatty acid | 10 | 1.56 | 0.64 | Long |
| Nandrolone phenylpropionate | |
C17β | Phenylpropanoic acid | Aromatic fatty acid | – (~6–7) | 1.48 | 0.67 | Long |
| Trenbolone acetate | |
C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.16 | 0.87 | Short |
| Trenbolone enanthated | |
C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | Long |
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative androgen/anabolic steroid content by weight (i.e., relative androgenic/anabolic potency). c = Duration by intramuscular or subcutaneous injection in oil solution. d = Never marketed. Sources: See individual articles. |
History
Edit
Drostanolone and drostanolone propionate were first described in 1959.[1][4] The related AAS oxymetholone and methasterone (methyldrostanolone) were first described in the same paper as well.[1] Drostanolone propionate was introduced for medical use in the United States in 1961 and in Europe shortly thereafter.[5]
Society and culture
Edit
Generic names
Edit
Drostanolone propionate is the generic name of the drug and its BANMTooltip British Approved Name, while dromostanolone propionate is the USANTooltip United States Adopted Name and USPTooltip United States Pharmacopeia; there is no INNTooltip International Nonproprietary Name for this form.[11][12][13] The generic name of the unesterified form of the drug is drostanolone or dromostanolone and the former is its INNTooltip International Nonproprietary Name, BANTooltip British Approved Name, and DCFTooltip Dénomination Commune Française while there is no USANTooltip United States Adopted Name.[11][12][13][2]
Brand names
Edit
Drostanolone propionate was marketed under a variety of brand names including Drolban, Masterid, Masteril, Masteron, Masterone, Mastisol, Metormon, Permastril, and Prometholone.[11][12][1]
Availability
Edit
Drostanolone propionate appears to no longer be marketed.[1][2] It was previously available in the United States, Europe, and Japan.[12][1] In Europe, it was specifically marketed in the United Kingdom, Germany, Belgium, France, Spain, Portugal, Italy, and Bulgaria.[12][1]
Legal status
Edit
Drostanolone propionate, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act.[6]
References
Edit
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 517–. ISBN 978-0-9828280-1-4.
- ^ a b c «Anabolic Agents». Drugs.com.
- ^ Kicman AT (June 2008). «Pharmacology of anabolic steroids». British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ^ a b Ringold HJ, Batres E, Halpern O, Necoechea E (1959). «Steroids. CV.12-Methyl and 2-Hydroxymethylene-androstane Derivatives». Journal of the American Chemical Society. 81 (2): 427–432. doi:10.1021/ja01511a040. ISSN 0002-7863.
- ^ a b William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1402–. ISBN 978-0-8155-1856-3.
- ^ a b Karch SB (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
- ^ «Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations». www.accessdata.fda.gov. Retrieved 2016-03-15.
- ^ «Drostanolone propionate (Masteron) administration — The Dose for Treating Breast Cancer». Masterone. 2020-01-24. Archived from the original on 2020-10-01. Retrieved 2021-02-23.
- ^ Solimini R, Rotolo MC, Mastrobattista L, Mortali C, Minutillo A, Pichini S, et al. (March 2017). «Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping». European Review for Medical and Pharmacological Sciences. 21 (1 Suppl): 7–16. PMID 28379599.
- ^ «Drostanolone (PIM 901)». www.inchem.org. Retrieved 2016-03-15.
- ^ a b c d e Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 652–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f g Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 377–. ISBN 978-3-88763-075-1.
- ^ a b Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 106–. ISBN 978-94-011-4439-1.
