ИНСТРУКЦИЯ ПО ПРИМЕНЕНИЮ ПОЛОСОК WangShi Manjing Flumethrin Strip
Важно! Перед применением необходимо протестировать препарат Манжинг на одной-трех пчелосемьях.
Действующее вещество: флуметрин 3.6 мг на полоску
Международное название: Flumethrin STRIP
Форма выпуска: желтая или оранжево-желтая полоска со слабым специфическим запахом.
Фармакологическое действие:
Противоакарацидное средство обладает контактной и желудочной токсичностью для клеща Варроа. Пчела, контактировавшая с препаратом, передает его другим пчелам, тем самым вызывая гибель клещей. Препарат безопасен для пчел и не обнаруживается в продукции пчел. Действие препарата проявляется через полчаса после помещения в улей. Через 21-28 дней полоски с препаратом вынимают из улья, чтобы не возникло резистентности клещей к этому препарату. Данное средство проявляет оптимальную эффективность и скорость уничтожения клещей и безопасен для окружающей среды.
Показания к применению:
Средство от варроатоза и акарапидоза пчел
Дозы и способ применения:
Одна полоска подвешиваются между рамками и через 7-10 дней добавить еще одну полосу. Через 28 дней снять все полосы и уничтожить их.
Отводкам, небольшим семьям можно дать одну полоску разделив на 2 части и подвесить в разных частях улья, а также увеличить или уменьшить дозу по кол-ву медовых сот.
Побочные действия: данный препарат не вызывает нежелательных реакций пчел при рекомендованных дозах.
Противопоказания: не использовать данный препарат при температуре ниже 12°С или если пчелосемье не хватает корма.
Не допускается совместное использование одновременно нескольких препаратов.
Особые указания:
- Препарат «Manjing Fluvalinate Strip» держать в прохладном месте вдали от пищевых продуктов.
- Обязательно протестируйте препарат на нескольких пчелосемьях (2-3) прежде чем обрабатывать всю пасеку
- Полоски препарата не брать голыми руками, используйте защитные перчатки, очки, респиратор.
- Промойте руки и кожу после работы с препаратом.
- Во время медосбора использовать препарат не рекомендуется из за меньшей эффективности и попадания препарата в мед.
Хранить в недоступном для детей месте и отдельно от продуктов и других химических веществ.
Форма выпуска: 20 полосок в пакете.
Срок годности: 2 года.
Условия хранения: в темном, прохладном месте.
Теги: Купить Флуметрин Манжинг ‘Wang Shi Manjing Flumethrin Strip’ (20 пластин) оптом и в розницу
Статья обновлена 02.12.2020
Краткое содержание:
Полоски для лечения пчел от варроатоза и акарапидоза
В настоящее время имеются действенные способы борьбы с клещами Varroa jacobsoni, предназначенные для профилактики и лечения заболевания с хорошим положительным результатом. Сегодня речь пойдет о препаратах Флувалидез, Флувалинат и Флуметрин. Будет описание препаратов и инструкции по их применению.
Флувалидез

Первый в нашем списке представитель полосок от клеща.
Форма выпуска и состав
Флувалидез производится в виде пластин (полос — называйте как хотите) из древесины, а точнее из шпона размером 200х20х0,8 мм. герметично упакованных в пакеты из фольги. В упаковке по 10 штук.

На каждой такой полоске нанесен:
- флувалинат — основное действующее вещество;
- эфирные масла чабреца, лаванды и розмарина — вспомогательные вещества природного происхождения.
Фармакологические свойства
Флувалидез – акарацидный и репеллентный препарат, воздействующий целенаправленно на нервную систему клеща Варроа, что влечёт в итоге его паралич и смерть.
Природные эфирные масла, содержащиеся в пластинках, обладают также бактерицидными, фунгицидными и инсектицидными свойствами. Опасности для самих пчёл и окружающей среды препарат не представляет. Не выявлено его следов и в готовой пчелиной продукции. Пластинка с веществом начинает действовать через полчаса после размещения внутри улья. Флувалидез проявляет оптимальную скорость уничтожения клещей и даёт хороший результат.
Производитель: ЗАО «Агробиопром», компания занимающаяся продажей препаратов, инвентаря, литературы по пчеловодству и семян.
Примерная цена: 150-175 руб. за упаковку (10 пластинок)
Инструкция по применению Флувалидеза для пчел
Если на пасеке все хорошо, то Флувалидез рекомендуется использовать как профилактическое средство от варратоза и акарапидоза 2 раза в год: весной (до размножения пчёл) и осенью (после забора мёда). Если есть сомнения или вы точно знаете, что ваши подопечные страдают от клеща, можно провести еще одну обработку зимой при несильных морозах.
Порядок действий
Пластинки размещают в разных точках улья в расчёте 2 полоски на 10-12 рамок. При меньшем количестве насекомых достаточно одной пластинки, зафиксированной в центре или поделённой пополам и расположенной в разных местах улья. Через 3-4 недели полоски с флувалидезом необходимо убрать в целях предотвращения устойчивости и невосприимчивости клещей к этому препарату и уничтожить,
Побочные эффекты
При соблюдении инструкции по применению, препарат безвреден, не вызывает нежелательных реакций. Вскрыть упаковку надо непосредственно на месте обработки. Флувалидез на качество мёда влияния не оказывает. Продукция, полученная после обработки средством, безвредна и может быть использована в пищу.
Условия хранения
Во избежание потери эффекта после использования, препарат Флувалидез необходимо хранить при t от 0°С до +25°С, в сухом месте, не допуская попадания солнечных лучей. Хранить вдали от детей и животных, продуктов питания и кормов. Срок годности 2 года
Флувалинат
Кандидат на обзор №2. Тоже представитель «полосочных» препаратов. Да, собственно, вся статья наша про пластинки-полоски от клеща. Флувалинат продается в виде полосок жёлтого или оранжево-жёлтого цвета с лёгким характерным запахом. 40 мг/пластинка, в пакете 10(20) пластинок.

Фармакологическое действие
Флувалинат — еще одно акарицидное средствo, только более сильное, с явной контактной и желудочной токсичностью, вызывающее отравление вредных насекомых при контакте с любой частью их тела. В основном их применяют против паразитов с колюще-сосущим ротовым аппаратом. Клещи Варроа Якобсони (возбудители варроатоза) и Акарапис Вуди (возбудитель акарапидоза) как раз подходят под это описание После контакта с препаратом, пчела распространяет его среди остальных пчёл. Вещество нарушает функции нервной системы паразитов, действуя на ионные каналы, после чего клещи массово гибнут. Для самих пчёл препарат опасности не представляет. Он не наблюдается при анализе в мёде пчёл.
Производитель: Sichuan Wangshi Animal Health Co., Ltd
Цену следует уточнять.
Инструкция по применению Флувалината для пчел
Одна пластинка Флувалината рассчитана на 7 медовых сот. Препарат подвешивают, не соприкасая с сотами, в диагональном направлении на рамках № 3,4 и № 6,7. Дейчтвие препарата начинается через 30 минут с момента помещения его в улье. Держать полоски флувалината в улье можно не более 3-4 недель. Затем убрать и уничтожить
Особые указания: запрещается использовать препарат в период сбора мёда пчёлами.Не советуется применять при смене места ульев (пчелы и так будут злые, а тут еще один раздражающий фактор)
Препарат китайский, поэтому, прежде чем радостно развешивать пластинки по всем уликам, лучше протестируйте его на паре семей. Флувалинат — лекарство быстрого действия и достаточно эффективное, несмотря на китайское происхождение. Повышенной опасности для окружающих не представляет, однако на вкус пробовать не советую.
Побочные действия и противопоказания
Препарат не вызывает побочных эффектов, если не отклоняться от инструкции по эксплуатации. Нельзя использовать средство при температуре минус 15°С или при нехватке сахара у пчёл. Для сохранения профилактического и лечебного эффекта не рекомендуется смешивать флувалинат с другими препаратами.
Условия хранения: в темном, прохладном, но не сыром месте.
Внимание! Средство сильно токсично для рыб и шелкопряда. Обеспечить недоступность детей к лекарству. Не допускается нахождение вблизи с продуктами питания.
К шелкопрядам я как-то равнодушен, а вот потравить рыбу в ближайшей речке не хочется. Короче, использованные полоски не выкидывать а сжигать в печи.
Срок годности: 4 года.
Важно!
В последние годы препарат проходит исследования в связи с обнаружением пестицидов в ульевых материалах. Несмотря на свою эффективность в борьбе с клещами Varroa jacobsoni, ученые утверждают, что он токсичен, и применять его следует только в крайних случаях, заменяя более «мягкими» аналогами.
Флуметрин Wangshi Manjing / Вангши Манжинг
Лекарственный препарат ветеринарного назначения в виде пластиковых полосок жёлтого цвета с узором , пропитанные флуметрином.
 Да, китайцы не парились с названием и назвали лекарство так же, как иосновное действующее вещество. Флуметрин последний в этой статье, но далеко не последний в списке противоклещевых лекрств. Полоски Флуметрина имеют слабый специфический запах.
Да, китайцы не парились с названием и назвали лекарство так же, как иосновное действующее вещество. Флуметрин последний в этой статье, но далеко не последний в списке противоклещевых лекрств. Полоски Флуметрина имеют слабый специфический запах.
Форма выпуска: 3,6mg/полоска, в герметичном пакете из фольги 10/20 пластин.
Фармакологические свойства
Основное действующее вещество — флуметрин, имеет ярковыраженный противоклещевой эффект, особенно в отношении Varroa, но и другим клещам он тоже не полезен. Флуметрин парализует нервную систему паразитов, в результате, вызывая их гибель.
Производитель: Sichuan Wangshi Animal Health Co., Ltd
Примерная цена 270 руб.
Флуметрин: инструкция по применению для пчел и дозировки
Полоску Флуметрина помещают в леток под углом 45°С, сроком на 10 дней, время от времени переворачивая пластинку. По истечении срока полоску заменяют на новую.
Если семья маленькая, то пластина разрезается пополам, помещается в подвешенном состоянии между рамами. Следует избегать попадания лекарственного вещества на соты. В случае тяжёлого состояния пчёл лечение продлевается до месяца, обновлять пластины рекомендуется каждую неделю.

Доза средства и кратность использования увеличивается или уменьшается в соответствии с учётом состояния медоносных пчёл.
Проводить обработку пчел за полмесяца до начала сбора мёда или после откачки меда, для предотвращения попадания лекарства в продукцию. Эффект применения лучший вне периода насиживания. Оптимальным временем является первая половина дня при солнечной погоде.
Превышать разовую дозу обработки не следует, это опасно для жизни пчёл и их личинок. При передозировке, дать пчелам 50% сахарного сиропа.
Меры предосторожности
Работая с препаратом, соблюдайте правила гигиены и безопасности. Перед началом обработки наденьте латексные перчатки, респиратор. В целях предупреждения вредного воздействия лекарства не курите, не пейте, не принимайте пищу не месте обработки. После окончания работы, вымойте руки с мылом, при попадании средства в глаза или на кожу, промойте большим количеством чистой воды. При проявлении симптомов аллергии срочно обратитесь к врачу.
Условия хранения
Средство, оставшееся после обработки, плотно упаковать. Место хранения средства не должно быть влажным. Не допускать попадания солнечных лучей. Хранить в отдельном от продуктов и кормов помещении, при t от 0°С до +50°С. Обеспечить недоступность детей к средству.
Срок годности: 2 года.
Описание
ИНСТРУКЦИЯ ПО ПРИМЕНЕНИЮ ПОЛОСОК WangShi Manjing Flumethrin Strip
Важно! Перед применением необходимо протестировать препарат Манжинг на одной-трех пчелосемьях.
Действующее вещество: флуметрин 3.6 мг на полоску
Международное название: Flumethrin STRIP
Форма выпуска: желтая или оранжево-желтая полоска со слабым специфическим запахом.
Фармакологическое действие:
Противоакарацидное средство обладает контактной и желудочной токсичностью для клеща Варроа. Пчела, контактировавшая с препаратом, передает его другим пчелам, тем самым вызывая гибель клещей. Препарат безопасен для пчел и не обнаруживается в продукции пчел. Действие препарата проявляется через полчаса после помещения в улей. Через 21-28 дней полоски с препаратом вынимают из улья, чтобы не возникло резистентности клещей к этому препарату. Данное средство проявляет оптимальную эффективность и скорость уничтожения клещей и безопасен для окружающей среды.
Показания к применению:
Средство от варроатоза и акарапидоза пчел
Дозы и способ применения:
Одна полоска подвешиваются между рамками и через 7-10 дней добавить еще одну полосу. Через 28 дней снять все полосы и уничтожить их.
Отводкам, небольшим семьям можно дать одну полоску разделив на 2 части и подвесить в разных частях улья, а также увеличить или уменьшить дозу по кол-ву медовых сот.
Побочные действия: данный препарат не вызывает нежелательных реакций пчел при рекомендованных дозах.
Противопоказания: не использовать данный препарат при температуре ниже 12°С или если пчелосемье не хватает корма.
Не допускается совместное использование одновременно нескольких препаратов.
Особые указания:
- Препарат «Manjing Fluvalinate Strip» держать в прохладном месте вдали от пищевых продуктов.
- Обязательно протестируйте препарат на нескольких пчелосемьях (2-3) прежде чем обрабатывать всю пасеку
- Полоски препарата не брать голыми руками, используйте защитные перчатки, очки, респиратор.
- Промойте руки и кожу после работы с препаратом.
- Во время медосбора использовать препарат не рекомендуется из за меньшей эффективности и попадания препарата в мед.
Хранить в недоступном для детей месте и отдельно от продуктов и других химических веществ.
Форма выпуска: 20 полосок в пакете.
Срок годности: 2 года.
Условия хранения: в темном, прохладном месте.
Поделиться ссылкой:
1 Introduction
The honeybee is a very important pollination insect (Kevan et al., 1990). It has huge economic value in global agriculture and plays an important role in maintaining ecological diversity (Potts et al., 2010). The Varroa mite is one of the most threatening pests in apiculture, causing huge economic losses to apiculture worldwide (Ghasemi et al., 2011). In honeybee colonies, Varroa mites damage the health of honeybees by sucking the fat and hemolymph from honeybee larvae and adults (Ramsey et al., 2019). At present, acaricides, such as flumethrin, are commonly used to control Varroa mites. Flumethrin is a second-generation pyrethroid insecticide used against Varroa mites through application within honeybee hives. Its main insecticidal mechanism is to delay the closure of ion channels and interfere with the normal physiological functions of the nervous systems of insects (Lund and Narahashi, 1981), causing symptoms such as excitement, convulsions, spasms, and even death (Catterall, 2001; Soderlund et al., 2002; Raymond-Delpech et al., 2005; Shafer et al., 2005). Flumethrin kills the mites but also affects the health of honeybees. When insecticides are used according to the manufacturer’s instructions, latent toxin interactions from insecticides and fungicides can affect the health of honeybee colonies and have sublethal effects in honeybees (Frost et al., 2012). In recent years, researchers have been paying attention to sublethal doses or concentrations of pesticides. Pesticides administered below the sublethal dose can still increase the susceptibility of honeybee to pathogens, cause certain damage to the learning, memory, and behavioral abilities of honeybees, and affect the development of the whole honeybee colony (El Hassani et al., 2005; Wu et al., 2011; Gill et al., 2012; Frost et al., 2012; Williamson et al., 2013; Doublet et al., 2015).
The adverse effects of flumethrin on honeybees through multiple life stages are well documented. Honeybee (A. mellifera) larvae exposed to flumethrin have been shown to have increased mortality, developmental failure, physiological changes, and low immunity in larval, pupal, and adult stages (Qi et al., 2020). Exposure of honeybee larvae to a sublethal dose of flumethrin has been shown to affect larval development, pupation and adult emergence rates, and the activities of key enzymes and related gene expression levels of A. mellifera (Niu, 2019). The toxic stress on honeybee larvae caused by flumethrin is alleviated by microorganisms, which play a role in intestinal barrier function and protect the larvae to some extent (Ramya et al., 2016). In addition, honeybee larvae can activate immune and detoxification systems to defend against the threat of increasing flumethrin concentrations (Yu et al., 2021). Flumethrin was shown to adversely affect the lifespan, immune function, and forage behavior of worker bees (A. mellifera) (Wu et al., 2023), and chronic exposure to sublethal concentrations of flumethrin was shown to negatively affect the development, olfactory learning, and memory of worker bees (A. mellifera; Li et al., 2022). The ingestion of a sublethal dose of sugar water containing flumethrin significantly affected the longevity and acquisition behavior of Apis cernana (Tan et al., 2013). Flumethrin has also been shown to affect the detoxification pathways of A. cernana (Jiang et al., 2016). Together, these studies show how flumethrin can affect honeybee development, immune and detoxification-related enzyme activity, behavior, and lifespan.
Due to the high lipophilic nature of flumethrin, its residues are often a problem in bee products (Niu, 2019). In one study, the residual concentrations of flumethrin detected in beeswax during a honey-flowing period ranged from 0.03 to 0.13 mg/kg, while residual concentrations as high as 3 mg/kg have been detected in older beeswax samples (Bogdanov, 2003; Johnson et al., 2010; Rosenkranz et al., 2010). A previous study reported a detection rate of flumethrin in honey samples of 75.3%, with a maximum concentration of 0.126 mg/kg (Yu et al., 2015). According to regulations put forth by the Chinese agricultural industry, the maximum residue limit (MRL) value of flumethrin in bee products is 0.01 mg/kg (Song et al., 2021). The European Union stipulates that flumethrin cannot be used with animals that provide milk for human consumption (Mutinelli, 2016). Recently, the issue of pesticide residues and their effects on human health have attracted more and more attention. This study aimed to investigate the impact of residual flumethrin levels in bee products on the physiological functions of honeybee larvae and newly emerged honeybees exposed to flumethrin during the larval stage. The findings from this study will fill the knowledge gap related to the chronic effects of flumethrin on honeybee development and provide new ideas for comprehensive risk assessments and application technology for acaricides in beehives.
2 Materials and methods
2.1 Honeybee and chemicals
Honeybees were obtained from the Honeybee Research Institute of Jiangxi Agricultural University (Jiangxi, China). The sucrose solution containing flumethrin was formulated according to the residual levels of flumethrin in bee products (Bogdanov, 2003; Johnson et al., 2010; Rosenkranz et al., 2010). Flumethrin (98.5% pure) was purchased from ANPEL Laboratory Technologies Inc. (Shanghai, China). Flumethrin was dissolved with acetone then diluted with a 50% sucrose solution (Xilong Scientific, Shantou, China) to get the following concentrations (1, 0.1, and 0.01 mg/L) for the three experimental groups. Sucrose solution (50%) was prepared for the control group.
2.2 Larva treatment
A new foundation was put into the experimental hive and the queen was kept to lay eggs on the new comb for 12 h when the new comb was being constructed. After the larvae hatched, they were equally divided into four groups with approximately 100 larvae per group. All larvae were from the same honeybee colony. From 2 days of age, experimental larvae were fed daily with 2 µl of sucrose solution containing flumethrin at concentrations of 1, 0.1, and 0.01 mg/L, while the control group was fed 50% sucrose solution until capped. The number of larvae was recorded daily before feeding. Because worker bees remove any dead larvae, the reduction in larvae count is equal to the number of dead larvae.
2.3 Enzyme content assay
Seven-day-old larvae (just capped) were frozen with liquid nitrogen and placed at −80°C for subsequent determination of related enzyme activities. Each sample contained a whole larva, and each group had five replicates. Protein concentrations were measured using a BCA Protein Assay Kit (BL521A, Biosharp, Anhui, China). The activities of glutathione sulfur transferase (GST), carboxylesterase (CarE), and mixed-functional oxidase (MFOs), as well as the total antioxidant capacity (T-AOC), were determined using kits A004-1-1, A33-1-1, H452-1, and A015-1, respectively (Nanjing Jiancheng Bioengineering Institute, China). All assays were performed according to the manufacturer’s instructions.
2.4 RNA isolation, library preparation, and sequencing
Honeybee larvae were treated according to the methods in Section 2.2 then were transferred to an incubator maintained at 34.5°C and 75% relative humidity for emerging once they had been capped for 12 days. The newly emerged honeybees were immediately frozen with liquid nitrogen and placed at -80°C for RNA isolation, library preparation, and sequencing. The head of the honeybee were put into an Eppendorf tube with 200 µl TransZoL Up (ER501-01, TransGen Biotech, Beijing, China) and fully ground with an electric micro-tissue crusher. Each tube was filled with 800 µl TransZoL Up. Total RNA was extracted as previously described (Qin et al., 2012). The RNA purity was determined using a nucleic acid protein tester (NanoPhotometerTM P300, Implen GmbH, Munich, Germany). Total RNA was reverse transcribed with a PrimeScript TR Reagent kit according to manufacturer’s instructions (RR047A, Takara Bio, Beijing, China), and the resulting complementary DNA (cDNA) was stored at -80°C. Sequencing work was completed by Novogene using the Illumina NovaSeq 6,000 sequencing platform (Illumina, San Diego, CA, United States).
2.5 Raw data acquisition and statistical analysis
After obtaining sequence reads, it was necessary to filter the raw data. Reads with adapter sequences, containing N (indicating that base information cannot be determined), or of low-quality (=20 bases representing more than 50% of the total read length) were removed. The expression level of each gene was normalized as fragments per kilobase of transcript sequence per millions mapped reads (FPKM) based on the gene length and the number of reads mapped to each gene (Mortazavi et al., 2008). An absolute value of log2Ratio ≥1 (twofold change) and false discovery ratio (FDR) ≤ 0.05 were set as significance thresholds and used to filter the differentially expressed genes (DEGs; Anders and Huber, 2012). ClusterProfile software (ver. 3.4.4) was used for Gene Ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of differential gene sets.
2.6 Validation by quantitative real-time PCR (qRT-PCR)
Four genes related to immunity, detoxification, and olfaction were analyzed by qRT-PCR with three biological and three technical replicates. These four genes were selected because honeybees resist insecticides through activation of their immune and detoxification pathways; insecticides also cause damage to the nervous system and impair the olfactory ability of honeybees. The gene-specific primers used in this study are presented in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control gene. Ct values of target and reference genes were collected, and the relative expression levels of each target gene were calculated (Huang et al., 2012).
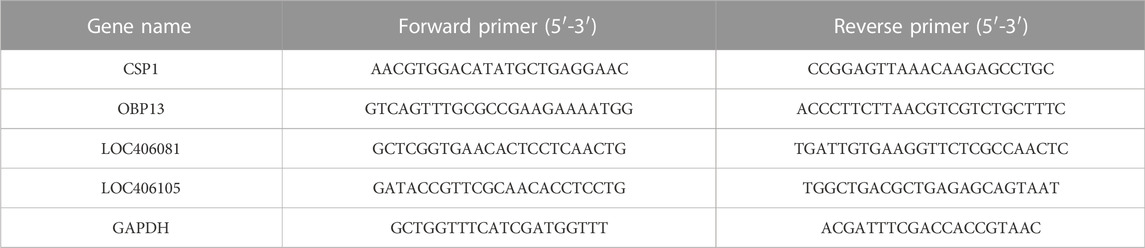
TABLE 1. Gene-specific primers used in quantitative real-time PCR.
2.7 Data analysis
The Statistical Package for the Social Sciences (SPSS) 17.0 was used to analyze the differences in enzyme activity and gene expression of the four groups. One-way analysis of variance (ANOVA) was used to compare the differences in enzyme activity and gene expression between the treatment and control groups. At p < 0.05, the least significant difference (LSD) test was used to determine differences between groups. GraphPad Prism 5 software (GraphPad Software, San Diego, CA, United States) was used to plot the survival curve of honeybee larvae and analyze the differences between each group by Kaplan-Meier analysis.
3 Results
3.1 Flumethrin affects honeybee larval survival
Honeybees were exposed to 1, 0.1, 0.01, or 0 mg/L of flumethrin during the larval stage from day 2 to day 5. The mortality rates of honeybee larvae in the 1 and 0.1 mg/L groups were significantly higher than those in the control group (1 mg/L vs control, p < 0.0001; 0.1 mg/L vs control, p < 0.0001; Figure 1); however, there was no significant difference in the mortality rates of honeybee larvae between the 0.01 mg/L and control groups (p = 0.3325).
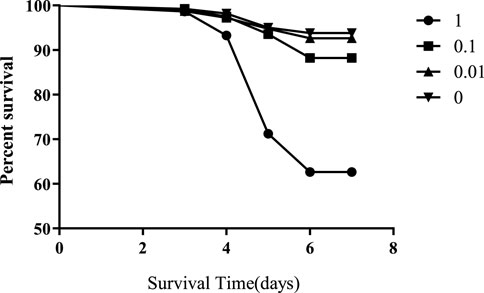
FIGURE 1. Effect of flumethrin on the survival of honeybee larvae.
3.2 Effects of flumethrin on immunity and detoxification-related enzyme activities of larvae
The activity trends of MFO, GST, CarE, and T-AOC in honeybee larvae exposed to different concentrations of flumethrin are shown in Figure 2. MFO content in the larvae increased gradually with an increase of flumethrin concentration (Figure 2A). The content of MFO in the 1 (17.90 ± 10.06 ng/g) and 0.1 mg/L groups (14.60 ± 10.28 ng/g) was significantly higher than in the 0.01 mg/L (8.344 ± 4.592 ng/g) and control groups (3.666 ± 2.473 ng/g; p < 0.05). There was no significant difference in T-AOC among the four groups (Figure 2B). The trends for CarE and GST (Figure 2C, Figure 2D) activities were similar. There was a significant decrease in the activities of GST and CarE in the 0.01 mg/L group compared with the control group (p < 0.05). On the other hand, GST and CarE activities in the 1 mg/L group were significantly higher than those in the control group (p < 0.05). There were no significant differences in the activities of GST and CarE between the 0.1 mg/L and control groups.
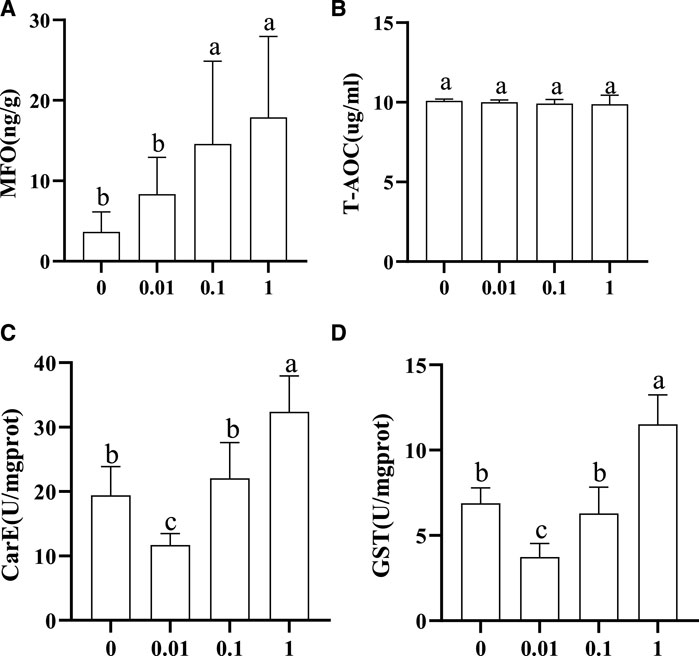
FIGURE 2. Effect of flumethrin on the content of immune and detoxification-related enzymes in honeybee larvae. The same letter above the bars means no significant difference. (A–D) represent the content of MFO, T-AOC, CarE, and Gst measured in each group, respectively.
3.3 Raw RNA-Seq data analysis
The transcriptome sequencing statistics for all samples are summarized in Supplemental Table S1. The raw reading included 4.03 × 107–4.79 × 107 reads. The clean reading segment included 3.98 × 107–4.71 × 107 reads after removing the invalid reading segments. Among the 11 libraries, more than 96.82% were Q20, and more than 91.83% were Q30. These results indicated that the data quality was good and was therefore used for subsequent analysis. The whole sequencing dataset was deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (accession number: PRJNA866287).
As shown in Figure 3A, with the increase in flumethrin concentration, the number of DEGs increased gradually. A total of 785 DEGs were identified between the 0.01 mg/L and the control groups. Of these, 260 were upregulated, and 525 were downregulated. A total of 1,083 DEGs were identified between the 0.1 mg/L and control groups, of which 538 DEGs were upregulated, and 545 DEGs were downregulated. In total, 2,214 DEGs were identified between the 1 mg/L and control groups, of which 1,006 DEGs were upregulated, and 1,208 DEGs were downregulated. The common DEGs are shown in Figure 3B and Table 2. There were 43 common DEGs, including loc102656552, Cox6c, rpl39, loc725432, loc100578551, and Apd-2, in the pesticide treatment group. In addition, as flumethrin concentration increased, more DEGs were produced. An additional 326 common DEGs, including olfactory-related genes (CSP2, CSP3, OBP17, OBP3) and an antioxidant gene (SOD1), were found in the 1 and 0.1 mg/L groups compared with the control group, as shown in Supplemental Table S2 (excluding the DEGs in Table S3). A total of 1,702 unique DEGs, including detoxification-related genes (GSTs1, loc406081) and an antioxidant gene (MsrA), were found in the 1 mg/L group compared with the control group (Supplemental Table S3).
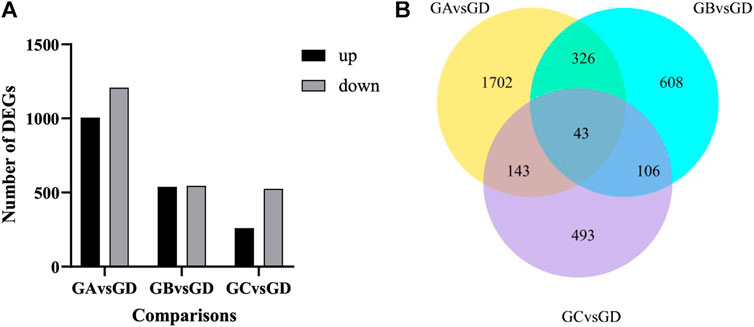
FIGURE 3. Effect of flumethrin on gene expression of newly emerged honeybees. (A) Numbers of DEGs of A. mellifera larvae treated with flumethrin. Up- and down-regulation indicates higher or lower expression of these genes in the treatment groups. (B) Venn analysis of DEGs. The sum of the numbers in each large circle represents the total number of DEGs in the comparative combinations, while the overlapping part of the circles represents the DEGs shared by the combinations. GA, GB, GC, and GD represent 1, 0.1, 0.01, and 0 mg/L groups, respectively.
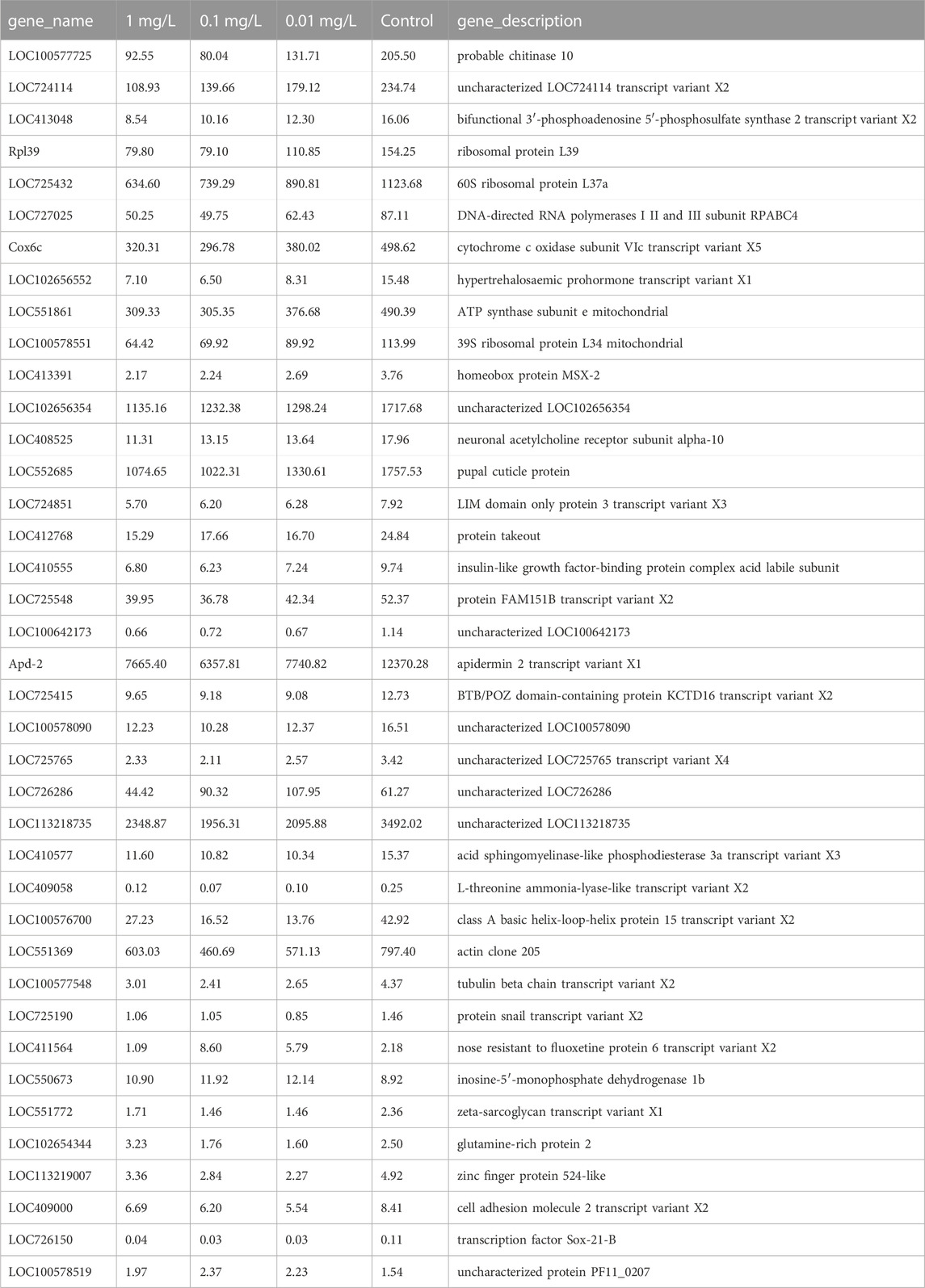
TABLE 2. The fragments per kilobase of exon per million mapped fragments (FPKM) of common DEGs between treated groups and control group.
The functions of the 43 common DEGs in the pesticide group were analyzed according to the GO database. The enriched terms in the biological process category included drug metabolic processes, oxidation-reduction processes, and ion transport. In the cellular component category, the enriched terms included plasma membrane part, plasma membrane protein complex, and membrane part. In the molecular function category, the enriched terms included receptor regulator activity, receptor ligand activity, and GTPase activity (Figure 4). In addition, these 43 common DEGs were related to enrichment in nine KEGG pathways, including ribosome function, purine metabolism, and oxidative phosphorylation (Figure 5).
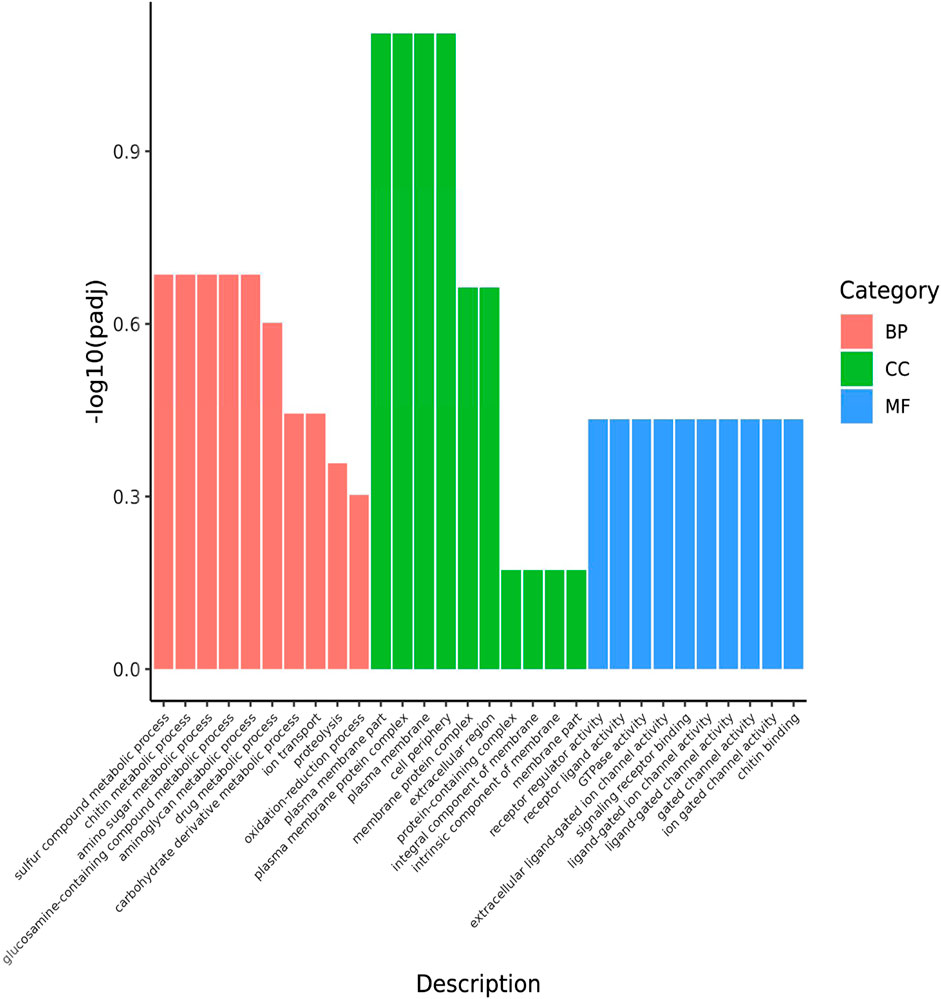
FIGURE 4. GO classification of DEGs shared by the 3 comparative combinations. The abscissa in the figure is GO terms; the ordinate is the significance level of GO Term enrichment, which is represented by -log10 (padj), and different colors represent different functional categories. BP: DEGs enriched for biological process; CC: DEGs enriched for cellular components; MF: DEGs enriched for molecular function. The same is below.
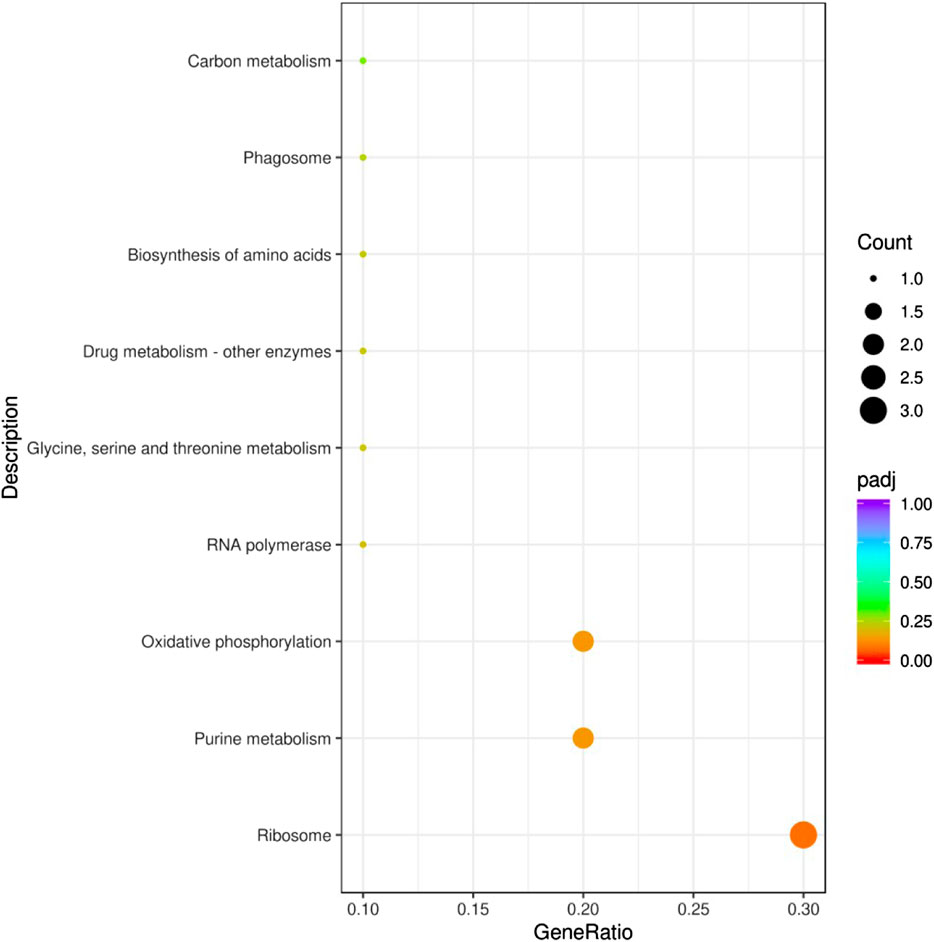
FIGURE 5. KEGG pathway enrichment analysis of DEGs. The abscissa in the figure is the ratio of the number of differential genes annotated to the KEGG pathway to the total number of differential genes. The ordinate is the KEGG pathway. Count: Number of DEGs annotated to the KEGG pathway. Padj: p-value corrected for multiple hypothesis testing.
3.4 Validation of target DEGs by qRT-PCR
The expression patterns of the selected 4 DEGs verified by qRT-PCR (Figure 6A) were similar to those of RNA-seq (Figure 6B) indicating that the abundance of the Illumina reads closely mirrored the actual expression levels of the DEGs. The validation results for the 4 DEGs indicated that the qPCR data correlated well with the transcriptome data and further confirmed the significance of flumethrin induced downregulation of genes involved in immunity, detoxification, and olfaction in the heads of honeybees.
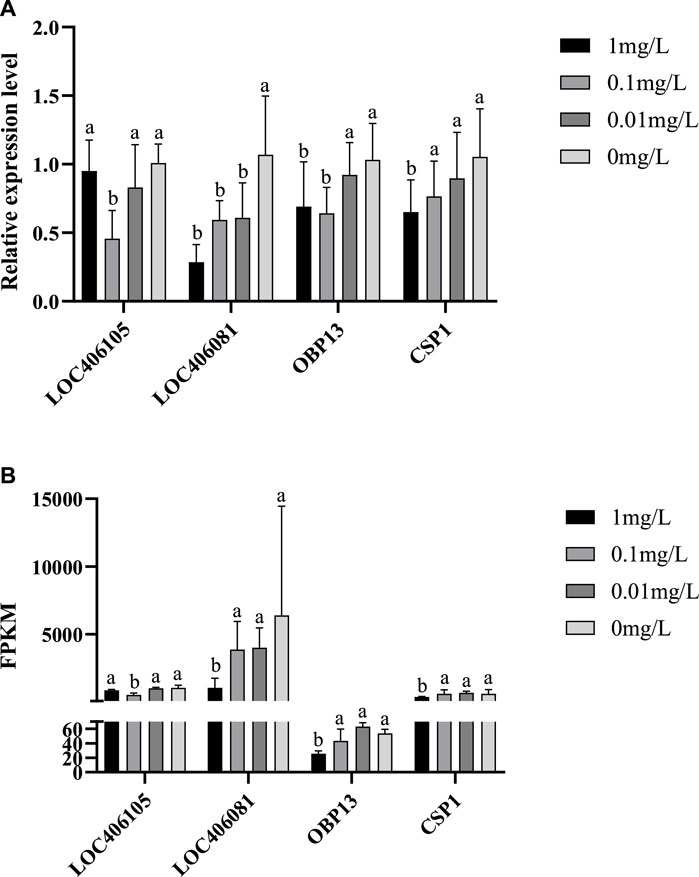
FIGURE 6. qRT-PCR verification (A) and RNA Seq data (B) of DEGs on the head of newly emerged honeybees in flumethrin-treated and control groups, respectively.
4 Discussion
Each honeybee goes through four life stages: egg, larva, pupa, and adult. The normal growth and development of larvae are the basis of the entire colony. However, larvae may be exposed to pesticides through activities such as living in contaminated hives and eating food containing pesticide residues, including royal jelly and honey (Tavares et al., 2017). In this study, the detection of immune and detoxification-related enzyme activities and RNA-Seq technology were used to investigate the toxic effects of flumethrin on honeybees.
There are three major immune and detoxification-related enzymes in insects, namely cytochrome P450 monooxygenase (P450), GST, and CarE (Claudianos et al., 2010). The core enzyme system of MFO determined in this study was the cytochrome P450 monooxygenase system. These enzymes have a direct impact on the detoxification and metabolism of insects and participate in the formation of drug resistance in insects (Enayati et al., 2005; Oakeshott et al., 2010). This study showed that the MFO activity increased with the increase in flumethrin concentration (Figure 2A). The MFO activity of larvae in the 1 and 0.1 mg/L groups was significantly higher than in the 0.01 mg/L and control groups, while there was no significant difference in larval MFO content between the 0.01 mg/L and control groups. These results indicate that residual levels of flumethrin (less than 0.01 mg/L) in the larval diet would not affect the activity of MFO. In contrast, MFO content could increase when honeybee larvae encounter residual flumethrin concentrations above 0.1 mg/L and could exert its detoxification effects to decompose toxic substances. With an increasing concentration of flumethrin, the activities of CarE and GST showed an initial decrease followed by a gradual increase (Figure 2C,D). It is possible that the activities of CarE and GST in the 0.01 mg/L group decreased due to using the larva’s CarE and GST enzymes neutralizing the negative effects of flumethrin. When the residual level of flumethrin in the larval diet was higher than 0.01 mg/L, more CarE and GST enzymes were produced to resist the toxic effect of pesticides; the concentration of these two enzymes rises to help prevent any harm to the body caused by flumethrin. Nielsen et al. (2000) measured the detoxification enzyme GST in larvae, pupae, and adult honeybees collected from flumethrin-treated colonies. They found significantly increased GST activities in the late fifth larval and pupal instars. T-AOC is an indicator of the antioxidant capacity in the reactive organism (Ghiselli et al., 2000). In this study, the content of T-AOC was not significantly different among the four treatment groups (Figure 2B), indicating that flumethrin might not affect the T-AOC of honeybee larvae. However, the mechanism should be further investigated.
The DEGs found in the present study reflect the multifaceted influence of flumethrin on honeybee physiology. There were huge differences in the number of DEGs in different dose groups compared with the control group (Figure 3). The number of DEGs increased with the increase in flumethrin concentration in the treatment groups, which showed that the higher the concentration of flumethrin, the greater the impact on and damage to honeybees. We found 43 common DEGs, including loc102656552, Cox6c, rpl39, loc725432, loc100578551, and Apd-2, in three of the treated groups. The expression level of the gene loc102656552, which is related to hypertrehalosemic hormone, was significantly downregulated. Hypertrehalosemic hormone can promote fat and glycogen decomposition in the body and increase the concentrations of lipids and trehalose in the blood (Rockstein, 1998). Trehalase converts trehalose stored in insects into glucose which is metabolized through glycolysis or the pentose phosphate pathway (Becker et al., 1996). The downregulated expression of loc102656552 could affect the synthesis of trehalose in honeybees, resulting a reduced trehalose content and affecting the growth, development, and resproduction of honeybees. Chen (2021) found that both carbendazim and Nosema ceranae could lead to the upregulated expression of genes encoding trehalose transporters and trehalase in honeybees, reducing blood trehalose levels. Cytochrome oxidase (Cox) plays an important role in the oxidative phosphorylation process of cells (Barazzoni et al., 2000). The expression of Cox6c was significantly downregulated after honeybee larvae received flumethrin, indicating that it could affect the process of oxidative phosphorylation, even at very low concentrations. The oxidative phosphorylation pathway is considered to be the most important biochemical process in animal cells. It mainly occurs in the inner membrane of mitochondria. As the final metabolic pathway of the respiratory chain, oxidative phosphorylation can provide adenosine triphosphate for a variety of basic cellular pathways, such as neural activity, material synthesis, and bioelectrogenesis (Byrne, 2009). The expression levels of ribosomal protein-related genes (rpl39, loc725432, and loc100578551) were significantly downregulated with flumethrin exposure. Ribosomal protein is an important part of the ribosome that plays an essential role in protein synthesis and translation (Byrne, 2009). Downregulated expression of these genes suggests that flumethrin exposure may affect energy metabolism and inhibit protein synthesis of honeybees. A recent study showed that sublethal doses of thiamethoxam caused the downregulation of genes related to oxidative phosphorylation and ribosomes in honeybees (Shi, 2020). The apidermin (APD) protein family is a newly discovered family of structural epidermal proteins in insects. Apd-2 belongs to the APD protein gene family and controls the main components of exoskeleton proteins in honeybees (Sun et al., 2012). Therefore, the downregulated expression of this epidermal gene may be a morphological manifestation of developmental delay. A previous study found that the expression levels of some epidermal protein family genes in the brain of honeybees exposed to carbendazim were downregulated (Fan, 2020). The present study showed that in all flumethrin-treated groups, genes involved in metabolism and biochemical processes in honeybee heads were significantly downregulated.
Annotation of enriched GO terms revealed that genes associated with the oxidation-reduction process and ion transport were significantly downregulated with exposure to flumethrin, which could lead to the generation of reactive oxidative species (ROS) (Figure 4). Increased oxidative stress was observed in neonicotinoid-exposed honeybees due to the excessive production of ROS (James and Xu, 2012). Iron plays an important role in redox reactions, the downregulation of genes involved in iron ion binding may indicate altered iron homeostasis in the brains of flumethrin-treated honeybees. A sublethal dose of imidacloprid has been shown to lead to downregulated expression levels of genes associated with the oxidation-reduction process and ion transport (Li et al., 2019). KEGG pathway analysis showed that most of the altered pathways were related to biological metabolic processes and biochemical reactions of the body (Figure 5). Together, these results show that flumethrin concentrations above 0.01 mg/L can affect the expression levels of genes related to biochemical processes and metabolism of substances in the heads of honeybees, affecting the growth and development of honeybees.
With the increasing flumethrin concentrations, the expression levels of olfactory-related genes, including CSP2, CSP3, OBP17, and OBP3, decreased significantly in the 1 and 0.1 mg/L groups compared to controls (Table S2). OBP17 and OBP3 belong to the odorant binding protein family, which are a class of water-soluble proteins that can bind odor molecules and help insects identify odors of a small molecular mass (Mu and Dong, 2004). CSP2 and CSP3 belong to the family of chemosensory proteins, whose main function is identifying, binding, and transporting non-volatile molecules (Xie et al., 2016). Imidacloprid can significantly reduce the expression of olfactory-related genes in the honeybee brain (Li et al., 2019). The down-regulation of olfactory-related genes may indicate an impaired olfactory function in honeybees exposed to flumethrin, leading to dysfunctional behaviors. The honeybee’s sense of smell is closely related to all of its life activities. For example, worker bees sense queen pheromones through smell, which helps to stabilize the whole colony; the drone senses the queen by smell and copulates with her (Xie et al., 2016). The changes in olfactory-related gene expression levels observed here may affect the recognition of informative materials and the exchange of information between honeybees, thus affecting the function of the entire colony. The antioxidant gene SOD1 was significantly downregulated in the 1 and 0.1 mg/L groups compared to controls. SOD1 can remove excess ROS in insects and protect the body from environmental stress (Mccord and Fridovich, 1969).
The higher the concentration of flumethrin in the treatment group, the higher the number of DEGs observed. Some DEGs, such as immune and detoxification-related genes (GST, loc406081) and antioxidant gene (MsrA), were differentially expressed only in the 1 mg/L group (Table S3). These three genes had significantly downregulated expression in the 1 mg/L group. GSTs are a multifunctional super gene family of enzymes that can metabolize various endogenous and exogenous substances and participate in the body’s detoxification of exogenous substances (e.g., pesticides). In the insect body, loc406081 can regulate the oxidation of glucose, produce energy and oxidation reactions to promote detoxification functions, and produce H202, which has an antibacterial function (Bucekova et al., 2014). Methionine sulfoxide reductase A (MsrA) belongs to the methylthionine sulfoxide reductase family, which are antioxidant and protein-repair factors in organisms with indirect antioxidant effects (Gong et al., 2012). Compared with the 0.01 and 0.1 mg/L groups, 1 mg/L flumethrin had a considerable impact on the expression levels of immunity, detoxification, and antioxidant genes of honeybees and a negative impact on the survival of honeybees.
5 Conclusion
This study showed that the residues of flumethrin at honey-relevant levels could affect the physiology of honeybee larvae and newly emerged worker honeybees (A. mellifera) exposed to flumethrin during the larval stage, and that with increases residual concentrations, the impact would be greater. Therefore, in beekeeping, we should pay close attention to the residues of flumethrin in bee products to ensure the health of honeybees and the production of high-quality bee products. In addition, the mechanisms through which honeybees can repair this damage after becoming adults remains to be further studied.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/,PRJNA866287.
Author contributions
X Wu conceived this research and designed the experiments. C Liu and X Wu wrote the manuscript. C Liu, Heyan Yang, Longtao Yu, and Yong Zhang contributed to the laboratory work. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China (31760714) and the Major Disciplines Academic and Technical Leader Projects of Jiangxi Province (20194BCJ22007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.1054769/full#supplementary-material
References
Anders S., Huber W. (2012). Differential expression of RNA-Seq data at the gene level–the DESeq package, 10. Heidelberg, Germany: European Molecular Biology Laboratory EMBL, f1000research.
Google Scholar
Barazzoni R., Short K. R., Nair K. S. (2000). Effects of aging on mitochondrial DNA copy number and cytochromec oxidase gene expression in rat skeletal muscle, liver, and heart. J. Biol. Chem. 275, 3343–3347. doi:10.1074/jbc.275.5.3343
PubMed Abstract | CrossRef Full Text | Google Scholar
Bogdanov S. (2003). Current status of analytical methods for the detection of residues in bee products. Apiacta 38, 190–197.
Google Scholar
Bucekova M., Valachova I., Kohutova L., Prochazka E., Klaudiny J., Majtan J. (2014). Honeybee glucose oxidase—its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 101, 661–670. doi:10.1007/s00114-014-1205-z
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen H. (2021). Effect of interactions between carbendazim and Nosema ceranae on the development of worker bees. Yangzhou: Yangzhou University. doi:10.27441/d.cnki.gyzdu.2021.000392
CrossRef Full Text | Google Scholar
Claudianos C., Ranson H., Johnson R. M., Biswas S., Schuler M. A., Berenbaum M. R., et al. (2010). A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 15, 615–636. doi:10.1111/j.1365-2583.2006.00672.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Doublet V., Labarussias M., de Miranda J. R., Moritz R. F., Paxton R. J. (2015). Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 17, 969–983. doi:10.1111/1462-2920.12426
PubMed Abstract | CrossRef Full Text | Google Scholar
El Hassani A. K., Dacher M., Gauthier M., Armengaud C. (2005). Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera). Pharmacol. Biochem. Behav. 82, 30–39. doi:10.1016/J.PBB.2005.07.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Fan L. (2020). Effects of carbendazim stress on the health of honeybee (Apis mellifera ligustica). Yangzhou: Yangzhou University. doi:10.27441/d.cnki.gyzdu.2020.000920
CrossRef Full Text | Google Scholar
Frost E. H., Shutler D., Hillier N. K. (2012). The proboscis extension reflex to evaluate learning and memory in honeybees (Apis mellifera): Some caveats. Naturwissenschaften 99, 677–686. doi:10.1007/s00114-012-0955-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Ghasemi V., Moharramipour S., Tahmasbi G. H. (2011). Acaricidal activity of essential oil from Mentha longifolia (Lamiaceae) against Varroa destructor (Acari: Varroidae) and its effect on Apis mellifera (Hym. Apidae) [J]. J. Entomological Soc. Iran 30 (2).
Google Scholar
Ghiselli A., Serafini M., Natella F., Scaccini C. (2000). Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 29, 1106–1114. doi:10.1016/s0891-5849(00)00394-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Gill R. J., Ramos-Rodriguez O., Raine N. E. (2012). Combined pesticide exposure severely affects individual-and colony-level traits in bees. Nature 491, 105–108. doi:10.1038/nature11585
PubMed Abstract | CrossRef Full Text | Google Scholar
Gong Z., Guo X., Xu B. (2012). Molecular cloning, characterisation and expression of methionine sulfoxide reductase A gene from Apis cerana cerana. Apidologie 43, 182–194. doi:10.1007/s13592-011-0099-4
CrossRef Full Text | Google Scholar
Huang Q., Kryger P., Conte Y. L., Moritz R. (2012). Survival and immune response of drones of a Nosemosis tolerant honey bee strain towards N. ceranae infections. J. Invertebr. Pathol. 109, 297–302. doi:10.1016/j.jip.2012.01.004
PubMed Abstract | CrossRef Full Text | Google Scholar
Jiang W. J., Xu-Jiang H. E., Wang Z. L., Yan W. Y., Zeng Z. J., Xiao-Bo W. U., et al. (2016). Cloning and expression analysis of cytochrome CYP9E2 gene in the Chinese honeybee, Apis cerana cerana. Acta Entomol. Sin. 59, 1050–1057.
Google Scholar
Johnson R. M., Ellis M. D., Mullin C. A., Frazier M. (2010). Pesticides and honey bee toxicity–USA. Apidologie 41, 312–331. doi:10.1051/apido/2010018
CrossRef Full Text | Google Scholar
Kevan P. G., Clark E. A., Thomas V. G. (1990). Insect pollinators and sustainable agriculture. Am. J. Alt. Ag. 5, 13–22. doi:10.1017/s0889189300003179
CrossRef Full Text | Google Scholar
Li Z., Yang H., Yu L., Liu C., Wu X. (2022). The negative effect of flumethrin stress on honey bee (Apis mellifera) worker from larvae to adults. Pestic. Biochem. Physiol. 188, 105289. doi:10.1016/j.pestbp.2022.105289
PubMed Abstract | CrossRef Full Text | Google Scholar
Li Z., Yu T., Chen Y., Heerman M., He J., Huang J., et al. (2019). Brain transcriptome of honey bees (Apis mellifera) exhibiting impaired olfactory learning induced by a sublethal dose of imidacloprid. Pestic. Biochem. Physiol. 156, 36–43. doi:10.1016/j.pestbp.2019.02.001
PubMed Abstract | CrossRef Full Text | Google Scholar
Mu L. F., Dong S. L. (2004). How do invertebrates recognize and discriminate chemical signals in the environment? Chin. J. Nat. 26, 305–309.
Google Scholar
Mutinelli F. (2016). Veterinary medicinal products to control Varroa destructor in honey bee colonies (Apis mellifera) and related EU legislation–an update. J. Apic. Res. 55, 78–88. doi:10.1080/00218839.2016.1172694
CrossRef Full Text | Google Scholar
Nielsen S. A., Brdsgaard C. J., Hansen H. (2000). Effects on detoxification enzymes in different life stages of honey bees (Apis mellifera L., hymenoptera: Apidae) treated with a synthetic pyrethroid (flumethrin). Altern. Lab. Anim. 28, 437–443. doi:10.1177/026119290002800312
CrossRef Full Text | Google Scholar
Niu X. (2019). Toxic effects of flumethrin on Apis mellifera ligustica L.(Hymenoptera:Apidae). Xinxiang: Henan Institute of Science and Technology.
Google Scholar
Oakeshott J., Claudianos C., Campbell P., Newcomb R., Russell R. (2010). Biochemical genetics and genomics of insect esterases. P. L. I. Gilbert, K. Iatrou, and S. S. Gill Editors. Compr. Mol. insect Sci. 5. doi:10.1016/b0-44-451924-6/00073-9
CrossRef Full Text | Google Scholar
Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., Kunin W. E. (2010). Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi:10.1016/j.tree.2010.01.007
PubMed Abstract | CrossRef Full Text | Google Scholar
Qi S., Zhu L., Wang D., Wang C., Chen X., Xue X., et al. (2020). Flumethrin at honey-relevant levels induces physiological stresses to honey bee larvae (Apis mellifera L.) in vitro. Ecotoxicol. Environ. Saf. 190, 110101. doi:10.1016/j.ecoenv.2019.110101
PubMed Abstract | CrossRef Full Text | Google Scholar
Qin Q. H., He X. J., Tian L. Q., Zhang S. W., Zeng Z. J. (2012). Comparison of learning and memory of Apis cerana and Apis mellifera. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 198, 777–786. doi:10.1007/s00359-012-0747-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Ramsey S. D., Ochoa R., Bauchan G., Gulbronson C., Mowery J. D., Cohen A., et al. (2019). Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. U. S. A. 116, 1792–1801. doi:10.1073/pnas.1818371116
PubMed Abstract | CrossRef Full Text | Google Scholar
Ramya S. L., Venkatesan T., Murthy K. S., Jalali S. K., Verghese A. (2016). Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, plutella xylostella (linnaeus), from India and its possible role in indoxacarb degradation. Braz. J. Microbiol. 47 (2), 327–336. doi:10.1016/j.bjm.2016.01.012
PubMed Abstract | CrossRef Full Text | Google Scholar
Raymond-Delpech V., Matsuda K., Sattelle B. M., Rauh J. J., Sattelle D. B. (2005). Ion channels: Molecular targets of neuroactive insecticides. Invert. Neurosci. 5, 119–133. doi:10.1007/s10158-005-0004-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Rockstein M. (1998). Insect biochemistry. Beijing: Science Press.
Google Scholar
Shafer T. J., Meyer D. A., Crofton K. M. (2005). Developmental neurotoxicity of pyrethroid insecticides: Critical review and future research needs. Environ. Health Perspect. 113, 123–136. doi:10.1289/ehp.7254
PubMed Abstract | CrossRef Full Text | Google Scholar
Shi T. (2020). Transcriptomic and metabolomic analysis of honeybees (Apis melifera ligustica) response to neonicotinoid insecticides. Hefei: Anhui Agricultural University. doi:10.26919/d.cnki.gannu.2020.000041
CrossRef Full Text | Google Scholar
Soderlund D. M., Clark J. M., Sheets L. P., Mullin L. S., Piccirillo V. J., Sargent D., et al. (2002). Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 171, 3–59. doi:10.1016/s0300-483x(01)00569-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Song Z., Zhang J., Li Y., Zhou J., Zhao W., Wang P., et al. (2021). “Comparative study on Maximum Residue Limits of pesticides in bee products in China and abroad,” in Quality and safety of agro-products, 78–83.
Google Scholar
Sun L., Huang Z., Zheng H., Ge Q., Gong L., Chen H. (2012). Characterization of three new members of the apidermin (apd) gene family from honeybees and sequence analysis of the insect APD family. Acta Entomol. Sin. 55, 12–23. CNKI:SUN:KCXB.0.2012-01-002
Google Scholar
Tan K., Shuang Y., Wang Z., Randolf M., Frederic M. P. (2013). Effect of flumethrin on survival and olfactory learning in honeybees. Plos One 8, e66295. doi:10.1371/journal.pone.0066295
PubMed Abstract | CrossRef Full Text | Google Scholar
Tavares D. A., Dussaubat C., Kretzschmar A., Carvalho S. M., Silva-Zacarin E. C. M., Malaspina O., et al. (2017). Exposure of larvae to thiamethoxam affects the survival and physiology of the honey bee at post-embryonic stages. Environ. Pollut. 229, 386–393. doi:10.1016/j.envpol.2017.05.092
PubMed Abstract | CrossRef Full Text | Google Scholar
Williamson S. M., Baker D. D., Wright G. A. (2013). Acute exposure to a sublethal dose of imidacloprid and coumaphos enhances olfactory learning and memory in the honeybee Apis mellifera. Invert. Neurosci. 13, 63–70. doi:10.1007/s10158-012-0144-7
PubMed Abstract | CrossRef Full Text | Google Scholar
Wu J. Y., Anelli C. M., Sheppard W. S. (2011). Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PloS one 6, e14720. doi:10.1371/journal.pone.0014720
PubMed Abstract | CrossRef Full Text | Google Scholar
Wu X., Li Z., Yang H., He X., Yan W., Zeng Z. (2023). The adverse impact on lifespan, immunity, and forage behavior of worker bees (Apis mellifera Linnaeus 1758) after exposure to flumethrin. Sci. Total Environ. 858, 160146. doi:10.1016/j.scitotenv.2022.160146
PubMed Abstract | CrossRef Full Text | Google Scholar
Xie C., Nie H., Su S. (2016). Advances in research on honey bee olfactory. Apic. China 67, 6. doi:10.3969/j.issn.0412-4367.2016.07.003
CrossRef Full Text | Google Scholar
Yu L., Liu F., Wu H., Tan H., Ruan X., Chen Y., et al. (2015). Flumethrin residue levels in honey from apiaries of China by high-performance liquid chromatography. J. Food Prot. 78, 151–156. doi:10.4315/0362-028X.JFP-14-297
PubMed Abstract | CrossRef Full Text | Google Scholar
Yu L., Yang H., Cheng F., Wu Z., Huang Q., He X., et al. (2021). Honey bee Apis mellifera larvae gut microbial and immune, detoxication responses towards flumethrin stress. Environ. Pollut. 290, 118107. doi:10.1016/j.envpol.2021.118107
PubMed Abstract | CrossRef Full Text | Google Scholar
Описание
Апиннум (флуметрин), 20 ПОЛОСОК — предназначен для лечения и профилактики варроатоза пчел (Varroa destructor).
Форма выпуска: Фольгированный полимерный пакет, содержащий 20 полосок картона фильтровального, размером 100 мм х 20 мм х 2.5 мм.
Состав: 1 полоска ветеринарного препарата «Апиннум» содержит действующее вещество: флуметрин — 4.15 мг.
Фармакологическое действие: Препарат «Апиннум» проявляет ярко выраженную инсектицидное и противопаразитарное действие, применяется при лечении варроатоза и при проведении комплексных профилактических мероприятий по клеща Varroa destructor. Субстанция флуметрин, действующее вещество препарата «Апиннум», является синтетическим аналогом природных веществ пиретроидов, которые проявляют активное репелентное, противопаразитарное и инсектицидное действие. Флуметрин активно действует на мембраны нервных клеток эктопаразитов, эффективно поражая их, что приводит к параличу и гибели клеща Varroa destructor. Использование полосок препарата «Апиннум» не приводит к возникновению новых популяций клещей, повышенной резистентности (у клеща нет привыкания).
Дозировка и способ применения: Применение ветеринарного препарата «Апиннум» для лечения пчелосемей, пораженных клещом Varroa destructor, проводят в течение всего пчеловодческого сезона, начиная с весны, после первого массового облет пчел, летом и осенью за 20 дней до начала периода основного медосбора или после его окончания. Перед подвешиванием полосок, дно улья покрывают чистым листом бумаги, смазанной маслом или вазелиновым маслом.
Обработку пчелиных семей, пораженных клещом Varroa destructor, проводят из расчета 2 полоски препарата «Апиннум» на 1 пчелиную семью, силой 10 плотно обсиженных пчелиных рамок, с размещением между 3-4 и 7-8 сотовыми рамками, и 1 пластину на пчелиную семью силой 5 сотовых рамок, с размещением в центре гнезда. В случае, когда степень поражения пчелосемьи клещом варроа высокая, рекомендуется дополнительно, через 7 дней после начала лечения, размещать в улье несколько полосок препарата «Апиннум», из расчета 1-2 полоски на 1 пчелиную семью, силой 10 сотовых рамок.
Срок лечебного действия полосок препарата «Апиннум» составляет 14 суток.
Внимание: Не использовать «Апиннум» одновременно с другими препаратами против варроатоза или нозематоза! Мед, полученный от пчелиных семей, обработанных препаратом «Апиннум», используют в пищу на общих основаниях. Упаковку препарата «Апиннум» следует открывать на пасеке, непосредственно перед применением.
Меры безопасности: При работе с препаратом принимать пищу, не пить, не курить. После работы тщательно вымыть руки с мылом.
Условия хранения: Препарат «Апиннум» следует хранить при температуре от 0 ° С до 20 ° С в упаковке производителя, в темном сухом прохладном месте, отдельно от пищевых продуктов и агрохимикатов.
Срок годности: 2 года.
Производство: Южная Корея
Вес: 170 грамм
БЕЗ АНТИБИОТИКА
Оплата и доставка
Оплата
НаличныеОплата наличными при получении
Доставка
СамовывозПочтовой службойКурьером (адресная доставка)
