Карисопродол
Carisoprodol
Фармакологическое действие
Карисопродол (каризопродол) — центральный миорелаксант. Точный механизм действия до конца не выяснен, оказывает седативное действие, расслабляет скелетную мускулатуру. Не действует непосредственно на скелетные мышцы, но влияет непосредственно на центральную нервную систему (ЦНС). У животных кариcопродол блокирует межнейрональную активность и подавляет передачу полисинаптических нейронов в спинной мозг и ретикулярную формацию головного мозга. Карисопродол также метаболизируется до мепробамата, который обладает анксиолитическим и седативным действием.
Фармакокинетика
Начало действия — быстрое, продолжительность действия — 4–6 часов. Время достижения максимальной плазменной концентрации (TCmax) — 90–120 минут.
Метаболизируется в печени с помощью CYP2C19 до активного метаболита (мепробамата). Связь с белками плазмы — карисопродол (<70 %), мепробамат (<25 %).
Период полувыведения (T½) — карисопродол: около 2 часов; мепробамат — около 10 часов.
Показания
Болезненный мышечный спазм при заболеваниях и травмах опорно-двигательного аппарата (в сочетании с физиотерапией и другими препаратами).
Противопоказания
- Повышенная чувствительность к карисопродолу;
- алкоголизм;
- миастения;
- печёночная и/или почечная недостаточность;
- эпилепсия;
- склонность к лекарственной зависимости;
- порфирия;
- беременность;
- период лактации;
- детский возраст до 5 лет.
Беременность и грудное вскармливание
Применение при беременности
Категория действия на плод по FDA — C.
Адекватных и строго контролируемых исследований по безопасности применения карисопродола при беременности не проведено. Неизвестно, оказывает ли препарат немедленное или отсроченное неблагоприятное воздействие на плод.
Исследования на животных выявили проникновение карисопродола через плацентарный барьер, неблагоприятные последствия воздействия препарата для роста плода и послеродовой выживаемости. Исследования на животных не дали адекватной оценки тератогенных эффектов. Исследования на животных показали снижение веса семенников и подвижности сперматозоидов при применении прапарата в дозе 1 200 мг/кг/день.
Применение карисопродола в период беременности противопоказано.
Применение в период грудного вскармливания
Специальных исследований по безопасности применения карисопродола в период грудного вскармливания не проведено.
Карисопродол и его метаболиты проникают в грудное молоко (существует риск седации новорождённого).
При необходимости применения препарата грудное вскармливание следует прекратить.
Способ применения и дозы
Внутрь.
Дети 5–12 лет: 6,25 мг/кг 4 раза в сутки.
Дети старше 12 лет и взрослые: 350 мг 4 раза в сутки.
Побочные действия
Со стороны нервной системы
Слабость, сонливость, головная боль, головокружение, бессонница, нарушение памяти и внимания, гипорефлексия, тревожность, парадоксальное возбуждение, парестезии, атаксия, нарушения зрения.
Со стороны сердечно-сосудистой системы
Брадикардия, аритмии, снижение артериального давления.
Со стороны пищеварительной системы
Сухость во рту, тошнота, рвота, диарея, боль в животе.
Прочие
Аллергические реакции.
Взаимодействие
Усиливает действие снотворных, анальгетиков.
Особые указания
Вызываемое снижение слюноотделения может привести к повышению риска развития пародонтоза, кандидоза и кариеса.
Карисопродол следует использовать только в течение коротких периодов (до 2 или 3 недель); доказательства эффективности для более длительного использования не были установлены.
Влияние на способность к вождению автотранспорта и управлению механизмами
Необходимо соблюдать осторожность при управлении автотранспортом и занятиями потенциально опасными видами деятельности, требующими повышенной концентрации внимания и скорости психомоторных реакций.
Классификация
-
АТХ
M03BA02
-
Фармакологическая группа
-
Категория при беременности по FDA
C
(риск не исключается)
Информация о действующем веществе Карисопродол предназначена для медицинских и фармацевтических специалистов, исключительно в справочных целях. Инструкция не предназначена для замены профессиональной медицинской консультации, диагностики или лечения. Содержащаяся здесь информация может меняться с течением времени. Наиболее точные сведения о применении препаратов, содержащих активное вещество Карисопродол, содержатся в инструкции производителя, прилагаемой к упаковке.
Generic name: carisoprodol [ kar-eye-soe-PROE-dole ]
Brand names: Soma, Vanadom
Drug class: Skeletal muscle relaxants
What is carisoprodol?
Carisoprodol is a muscle relaxer that blocks pain sensations between the nerves and the brain.
Carisoprodol is used together with rest and physical therapy to treat skeletal muscle conditions such as pain or injury.
Carisoprodol should only be used for short periods (up to two or three weeks) because there is no evidence of its effectiveness in long term use and most skeletal muscle injuries are generally of short duration. Carisoprodol is considered a controlled substance in the United States.
Warnings
You should not take carisoprodol if you have porphyria (a genetic enzyme disorder that causes symptoms affecting the skin or nervous system).
Carisoprodol may be habit-forming. Never share this medicine with another person. Misuse of habit-forming medicine can cause addiction, overdose, or death.
Carisoprodol can cause side effects that may impair your thinking or reactions. Be careful if you drive or do anything that requires you to be awake and alert. Avoid drinking alcohol. It can increase drowsiness and dizziness caused by this medicine.
You may have withdrawal symptoms when you stop using this medicine after using it over a long period of time. Do not stop using this medication suddenly without first talking to your doctor. You may need to use less and less before you stop the medication completely.
Before taking this medicine
You should not use this medicine if you are allergic to carisoprodol or meprobamate, or if you have:
-
porphyria (a genetic enzyme disorder that causes symptoms affecting the skin or nervous system).
Tell your doctor if you have ever had:
-
liver disease;
-
kidney disease; or
-
a seizure.
It is not known whether this medicine will harm an unborn baby. Tell your doctor if you are pregnant.
Carisoprodol can pass into breast milk and may cause drowsiness in a nursing baby. Tell your doctor if you are breast-feeding.
This medicine is not approved for use by anyone younger than 16 years old.
Older adults may be more sensitive to the effects of this medicine.
How should I take carisoprodol?
Take carisoprodol exactly as it was prescribed for you. Follow all directions on your prescription label and read all medication guides or instruction sheets.
Carisoprodol may be habit-forming. Misuse can cause addiction, overdose, or death. Selling or giving away this medicine is against the law.
Carisoprodol is usually taken 3 times per day and at bedtime and should be only be taken for 2 or 3 weeks.. Call your doctor if your symptoms do not improve, or if they get worse. Follow your doctor’s dosing instructions very carefully.
Do not stop using this medicine suddenly after long-term use, or you could have unpleasant withdrawal symptoms. Ask your doctor how to safely stop using this medicine.
Carisoprodol is only part of a complete program of treatment that may also include rest, physical therapy, or other pain relief measures. Follow your doctor’s instructions.
Store at room temperature away from moisture and heat.
Keep track of your medicine. Carisoprodol is a drug of abuse and you should be aware if anyone is using it improperly or without a prescription.
Dosing information
Usual Adult Dose for Muscle Spasm:
250 to 350 mg orally 3 times a day and at bedtime
Duration of therapy: Up to 2 to 3 weeks
Comments:
-This drug should only be used for short periods (up to 2 or 3 weeks) as there is inadequate evidence of effectiveness for more prolonged use and acute, painful musculoskeletal conditions are generally of short duration.
Use: For the relief of discomfort associated with acute, painful musculoskeletal conditions
What happens if I miss a dose?
Take the medicine as soon as you can, but skip the missed dose if it is almost time for your next dose. Do not take two doses at one time.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222. An overdose of carisoprodol can be fatal, especially if you take it with alcohol or with other drugs that can slow your breathing.
Overdose symptoms may include vision problems, confusion, hallucinations, muscle stiffness, loss of coordination, weak or shallow breathing, fainting, seizure, or coma.
What to avoid
Do not drink alcohol. Dangerous side effects or death could occur.
Avoid driving or hazardous activity until you know how this medicine will affect you. Dizziness or drowsiness can cause falls, accidents, or severe injuries.
Carisoprodol side effects
Get emergency medical help if you have signs of an allergic reaction to carisoprodol: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
Stop using this medicine and call your doctor at once if you have:
-
a seizure (convulsions); or
-
high levels of serotonin in the body agitation, hallucinations, fever, sweating, shivering, fast heart rate, muscle stiffness, twitching, loss of coordination, nausea, vomiting, diarrhea.
Common carisoprodol side effects may include:
-
drowsiness;
-
dizziness; or
-
headache.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What other drugs will affect carisoprodol?
Sometimes it is not safe to use certain medications at the same time. Some drugs can affect your blood levels of other drugs you take, which may increase side effects or make the medications less effective.
Using carisoprodol with other drugs that make you sleepy or slow your breathing can cause dangerous side effects or death. Ask your doctor before using opioid medication, a sleeping pill, a muscle relaxer, or medicine for anxiety or seizures.
Many drugs can interact with carisoprodol. This includes prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible interactions are listed here. Tell your doctor about all your current medicines and any medicine you start or stop using.
Popular FAQ
Caridoxen is a brand name of a combination medicine available in Mexico that contains naproxen (250mg) and carisoprodol (200mg) which may be used to control pain and inflammation and relieve muscle spasms. Caridoxen may be used as a muscle relaxant. Continue reading
Further information
Remember, keep this and all other medicines out of the reach of children, never share your medicines with others, and use this medicine only for the indication prescribed.
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Medical Disclaimer
Copyright 1996-2023 Cerner Multum, Inc. Version: 5.01.

| Carisoprodol | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||
| Chemical Nomenclature | |||||||||||||||||||
| Common names | Carisoprodol, Soma | ||||||||||||||||||
| Substitutive name | Isopropyl meprobamate | ||||||||||||||||||
| Systematic name | [2-(Carbamoyloxymethyl)-2-methylpentyl] N-propan-2-ylcarbamate | ||||||||||||||||||
| Class Membership | |||||||||||||||||||
| Psychoactive class | Depressant | ||||||||||||||||||
| Chemical class | Carbamate | ||||||||||||||||||
| Routes of Administration | |||||||||||||||||||
|
WARNING: Always start with lower doses due to differences between individual body weight, tolerance, metabolism, and personal sensitivity. See responsible use section.
DISCLAIMER: PW’s dosage information is gathered from users and resources for educational purposes only. It is not a recommendation and should be verified with other sources for accuracy. |
|||||||||||||||||||
| Interactions | |||||||||||||||||||
| Stimulants | |||||||||||||||||||
| Depressants | |||||||||||||||||||
| Dissociatives |
Carisoprodol, also known by the brand name Soma, is a carbamate sedative-hypnotic. Carisoprodol is used medically as a centrally-acting muscle relaxant, anxiolytic and hypnotic for the short-term treatment of insomnia. Carisoprodol also has weak analgesic effects. Carisoprodol is sometimes found in formulations also containing caffeine and acetaminophen. Carisoprodol produces similar effects to barbiturates. Carisoprodol acts as a prodrug to meprobamate, meaning it is metabolized to meprobamate when it enters the body.
Carisoprodol, like barbiturates, has been primarily replaced by benzodiazepines due to a larger therapeutic window, having less severe adverse effects and being safer in overdose.
Chemistry
Chemically, carisoprodol is classified as a carbamate. It is extremely similar in structure to meprobamate, the only difference being an isopropyl group bonded to an amine group. Carbamates are derivatives of carbamic acid. The empirical formula is carisoprodol is C12H24N2O4 and has a molar mass of 260.33 grams per mole.
Pharmacology
Carisoprodol is a prodrug that is metabolized to meprobamate. The precise mechanism of meprobamate is not completely understood. However it is believed that meprobamate acts similarly to benzodiazepines and barbiturates, acting as a positive allosteric modulator of a GABAA receptor[2]. Unlike barbiturates and benzodiazepines, in animal studies meprobamate has been shown to retain most of its effects without having gamma-aminobutyric acid present[2]. Meprobamate has also been noted to be an adenosine reuptake inhibitor, making it unique among hypnotics[3].
Carisoprodol is metabolized by the cytochrome P450 2C19 enzyme in the liver and has a biological half-life of about two hours. Carisoprodol and its metabolites are excreted by the kidneys in urine.
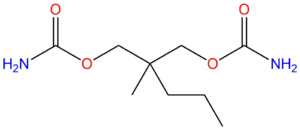
Carisoprodol is a prodrug to meprobamate (pictured).
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
-
- Sedation — In terms of energy level alterations, this drug has the potential to be extremely sedating and often results in an overwhelmingly lethargic state. However, unlike benzodiazepines, carisoprodol causes direct GABA-A agonism at higher doses, resulting in more significant sedation in comparison. It can be described as more akin to barbiturates.
- Motor control loss
- Muscle relaxation
- Dizziness
- Respiratory depression
- Physical euphoria — This effect generally occurs only at heavier doses, but may be present at lower doses as well. The euphoria felt on carisoprodol is significantly stronger than that felt on benzodiazepines.
- Decreased libido
- Pain relief — Compared to other agents such as opioids, this effect is generally considered to be quite weak.
-
- Acuity suppression — Like many depressants, high doses of carisoprodol may cause blurred vision.
-
- Anxiety suppression
- Cognitive euphoria — Compared to most other depressants, this effect is particularly strong.
- Thought deceleration
- Analysis suppression
- Disinhibition
- Amnesia
- Language suppression — At higher doses, carisoprodol is known cause slurred speech.
- Compulsive redosing
- Delusions of sobriety — This is the false belief that one is perfectly sober despite obvious evidence to the contrary such as severe cognitive impairment and an inability to fully communicate with others. It most commonly occurs at heavy dosages.
Experience reports
There are currently no anecdotal reports which describe the effects of this compound within our experience index. Additional experience reports can be found here:
- Erowid Experience Vaults: Carisoprodol
Toxicity and harm potential
Carisoprodol likely has moderate toxicity relative to dose. However, carisoprodol is potentially lethal when mixed with depressants like alcohol or opioids. Carisoprodol has been taken off the market is several countries such as Sweden and Indonesia due to side effects and abuse.
It is strongly recommended that one use harm reduction practices when using this drug.
Tolerance and addiction potential
Carisoprodol is extremely physically and psychologically addictive. Carbamate withdrawal, like barbiturate withdrawal, is medically serious and can potentially cause a life-threatening withdrawal syndrome that can cause seizures, psychosis, and death. Drugs which lower the seizure threshold such as tramadol and amphetamine should be avoided during withdrawal.
Tolerance will develop to the sedative-hypnotic effects of carisoprodol after prolonged use. It is unknown exactly how long it takes for tolerance to reach baseline.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Depressants (1,4-Butanediol, 2M2B, alcohol, benzodiazepines, barbiturates, GHB/GBL, methaqualone, opioids) — This combination potentiates the muscle relaxation, amnesia, sedation, and respiratory depression caused by one another. At higher doses, it can lead to a sudden, unexpected loss of consciousness along with a dangerous amount of depressed respiration. There is also an increased risk of suffocating on one’s vomit while unconscious. If nausea or vomiting occurs before a loss of consciousness, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Dissociatives — This combination can unpredictably potentiate the amnesia, sedation, motor control loss and delusions that can be caused by each other. It may also result in a sudden loss of consciousness accompanied by a dangerous degree of respiratory depression. If nausea or vomiting occurs before consciousness is lost, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Stimulants — Stimulants mask the sedative effect of depressants, which is the main factor most people use to gauge their level of intoxication. Once the stimulant effects wear off, the effects of the depressant will significantly increase, leading to intensified disinhibition, motor control loss, and dangerous black-out states. This combination can also potentially result in severe dehydration if one’s fluid intake is not closely monitored. If choosing to combine these substances, one should strictly limit themselves to a pre-set schedule of dosing only a certain amount per hour until a maximum threshold has been reached.
Legal status
In most jurisdictions, carisoprodol is considered a prescription-only and/or controlled drug.
- Germany: Carisoprodol is a prescription medicine, according to Anlage 1 AMVV.[4]
- United States: In the United States, carisoprodol is a Schedule IV Controlled Substance[5]. Therefore, it is prescription-only and anyone caught in possession of the substance with or without intent to distribute is punishable by law.
External links
- Carisoprodol (Wikipedia)
- Carisoprodol (Erowid Vault)
- Carisoprodol (TiHKAL / Isomer Design)
References
- ↑ Risks of Combining Depressants — TripSit
- ↑ 2.0 2.1 Rho, J. M., Donevan, S. D., Rogawski, M. A. (1 March 1997). «Barbiturate-Like Actions of the Propanediol Dicarbamates Felbamate and Meprobamate». Journal of Pharmacology and Experimental Therapeutics. 280 (3): 1383–1391. ISSN 0022-3565.
- ↑ Phillis, J. W., Delong, R. E. (1 June 1984). «A purinergic component in the central actions of meprobamate». European Journal of Pharmacology. 101 (3–4): 295–297. doi:10.1016/0014-2999(84)90174-2. ISSN 0014-2999.
- ↑ AMVV — Verordnung über die Verschreibungspflicht von Arzneimitteln
- ↑ DEA Scheduled Drugs | https://www.deadiversion.usdoj.gov/schedules/orangebook/e_cs_sched.pdf
Произношение
Общее название: carisoprodol (кар-глазное soe PROE dole)
Названия марок:Сома, Ванадом

Carisoprodol — расслабляющий мышцы, который блокирует болевые ощущения между нервами и мозгом.
Carisoprodol используется вместе с отдыхом и физической терапией для лечения состояний скелетных мышц, таких как боль или травма.
Carisoprodol также может использоваться для целей, не указанных в данном руководстве.
Вы не должны принимать carisoprodol, если у вас есть порфирия (генетическое расстройство фермента, которое вызывает симптомы, влияющие на кожу или нервную систему).
Каризопродол может быть привыканием. Никогда не делитесь этим лекарством с другим человеком. Неправильное использование привыкающей медицины может вызвать зависимость, передозировку или смерть.
Слайд-шоу Тенденции моды, которые могут повлиять на ваше здоровье
Carisoprodol может вызвать побочные эффекты, которые могут ухудшить ваше мышление или реакции. Будьте осторожны, если вы едете или делаете все, что требует от вас бодрствования и тревоги. Избегайте употребления алкоголя. Это может увеличить сонливость и головокружение, вызванные этим лекарством.
У вас могут быть симптомы отмены, когда вы прекратите использовать каризопродол после его использования в течение длительного периода времени. Не прекращайте использовать этот препарат внезапно, не обсудив сначала своего врача. Возможно, вам придется использовать все меньше и меньше, прежде чем полностью прекратить прием лекарства.

Вы не должны использовать это лекарство, если у вас аллергия на каризопродол или мепробамат, или если у вас есть:
-
Порфирия (генетическое расстройство фермента, которое вызывает симптомы, влияющие на кожу или нервную систему).
Чтобы убедиться, что это лекарство безопасно для вас, сообщите своему врачу, если у вас есть:
-
болезнь печени;
-
Болезнь почек; или
-
История судорог.
Неизвестно, вредит ли это лекарство нерожденному ребенку. Расскажите своему врачу, если вы беременны.
Carisoprodol может перейти в грудное молоко и может нанести вред грудному ребенку. Расскажите своему врачу, если вы кормите ребенка грудью.
Это лекарство не одобрено для использования кем-либо моложе 16 лет.
Пожилые люди могут быть более чувствительны к воздействию этого лекарства.
Возьмите carisoprodol точно так, как это было предписано для вас. Следуйте всем указаниям на этикетке рецепта. Не принимайте это лекарство в больших или меньших количествах или дольше, чем рекомендуется.
Каризопродол обычно принимают 3 раза в день и перед сном. Следуйте инструкциям своего врача очень осторожно.
Это лекарство следует использовать только на короткое время; До 2 или 3 недель, если ваш врач не сообщит вам об обратном.
Каризопродол может быть привыканием. Никогда не делитесь этим лекарством с другим человеком, особенно с наркоманией или наркоманией. Держите лекарство в месте, где другие не могут добраться до него. Продажа или раздача этого лекарства противоречит закону.
Неправильное использование привыкающей медицины может вызвать зависимость, передозировку или смерть.
Не прекращайте использовать это лекарство внезапно после длительного использования, или у вас могут быть неприятные симптомы отмены. Попросите вашего врача, как безопасно прекратить использование этого лекарства.
Carisoprodol является лишь частью полной программы лечения, которая может также включать отдых, физическую терапию или другие меры по обезболиванию. Следуйте инструкциям вашего врача.
Хранить при комнатной температуре вдали от влаги и тепла.
Следите за количеством лекарства, используемого в каждой новой бутылке. Carisoprodol является наркотиком злоупотребления, и вы должны знать, кто использует ваше лекарство неправильно или без рецепта.
См. Также: Информация дозирования (более подробно)

Примите пропущенную дозу, как только вспомните. Пропустите пропущенную дозу, если это почти время для вашей следующей запланированной дозы. Не принимайте дополнительное лекарство, чтобы восполнить пропущенную дозу.
Читайте так же про препарат Halaven.
Обратитесь за неотложной медицинской помощью или позвоните в справочную строку Poison по телефону 1-800-222-1222. Передозировка carisoprodol может быть фатальной, особенно если вы принимаете это лекарство с алкоголем или другими препаратами, которые могут замедлить ваше дыхание.
Симптомы передозировки могут включать проблемы со зрением, путаницу, галлюцинации, мышечную жесткость, слабое или неглубокое дыхание, обморок или кома.
Каризопродол может ухудшить ваше мышление или реакцию. Избегайте вождения или управления машиной, пока вы не узнаете, как это лекарство повлияет на вас. Головокружение или тяжелая сонливость могут стать причиной падений или других аварий.
Не употребляйте алкоголь. Опасные побочные эффекты или смерть могут возникать, когда алкоголь сочетается с этим лекарством.
Читайте так же про препарат Bromday.
Получите экстренную медицинскую помощь, если у вас есть признаки аллергической реакции на каризопродол : ульи; Затрудненное дыхание; Отек лица, губ, языка или горла.
Прекратите использовать это лекарство и сразу же обратитесь к врачу, если у вас есть:
-
Приступ (судороги); или
-
Высокий уровень серотонина в организме — возбуждение, галлюцинации, лихорадка, быстрый сердечный ритм, сверхактивные рефлексы, тошнота, рвота, диарея, потеря координации, обморок.
Общие побочные эффекты каризопродола могут включать:
-
сонливость;
-
головокружение; или
-
Головная боль.
Это не полный список побочных эффектов, и другие могут возникнуть. Спросите у своего доктора о побочных эффектах. Вы можете сообщить о побочных эффектах FDA на уровне 1-800-FDA-1088.
См. Также: Побочные эффекты (более подробно)
Обычная доза для взрослых для судорог мышц:
250-350 мг устно 3 раза в день и перед сном
Продолжительность терапии: до 2 — 3 недель
Комментарии:
-Этот препарат следует использовать только на короткие периоды (до 2 или 3 недель), так как есть недостаточные доказательства эффективности для более длительного использования и острые, болезненные скелетно-мышечные условия, как правило, кратковременны.
Применение: для облегчения дискомфорта, связанного с острыми, болезненными скелетно-мышечными условиями
Принимая это лекарство с другими препаратами, которые заставляют вас спать или замедлять дыхание, вы можете вызвать опасные побочные эффекты или смерть. Попросите вашего врача, прежде чем принимать снотворное, лекарство от наркотической боли, лекарство от кашля по рецепту, расслабляющий мышцы или лекарство для беспокойства, депрессии или судорог.
Расскажите своему врачу обо всех ваших текущих лекарствах и о том, что вы начинаете или прекращаете использовать, особенно:
-
Флуоксетин или флувоксамин;
-
рифампицин;
-
Зверобой;
-
Противогрибковая медицина — кетоконазол, вориконазол;
-
Седативно- диазепам, альпразолам, лоразепам, валиум, ксанакс и другие;
-
Судорожная медицина — карбамазепин, окскарбазепин; или
-
Желудочные кислотные восстановители — эзомепразол, лансопразол, омепразол, пантопразол, нексий, прилосец.
Этот список не является полным. Другие препараты могут взаимодействовать с каризопродолом, включая лекарственные средства, отпускаемые по рецепту и без рецепта, витамины и растительные продукты. Не все возможные взаимодействия перечислены в данном руководстве по лекарствам.
- Ваш фармацевт может предоставить дополнительную информацию о каризопродоле.
Carisoprodol, sold under the brand name Soma among others, is a medication used for musculoskeletal pain.[3] Use is only approved for up to three weeks.[3] Effects generally begin within half an hour and last for up to six hours.[3] It is taken orally.[3]
 |
|
| Clinical data | |
|---|---|
| Pronunciation | kahr-EYE-suh-PROH-dol |
| Trade names | Soma, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682578 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth |
| Drug class | Carbamate |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 60% |
| Metabolism | Liver (CYP2C19-mediated) |
| Metabolites | Meprobamate |
| Onset of action | Rapid (30 minutes[2][failed verification]) |
| Elimination half-life | 2.5 hours [12 hours[nb 1]] |
| Excretion | Kidney |
| Identifiers | |
|
IUPAC name
|
|
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.001.017 |
| Chemical and physical data | |
| Formula | C12H24N2O4 |
| Molar mass | 260.334 g·mol−1 |
| 3D model (JSmol) |
|
|
SMILES
|
|
|
InChI
|
|
| (verify) |
Common side effects include headache, dizziness, and sleepiness.[3] Serious side effect may include addiction, allergic reactions, and seizures.[3] In people with a sulfa allergy certain formulations may result in problems.[3] Safety during pregnancy and breastfeeding is not clear.[3][4] How it works is not clear.[3] Some of its effects are believed to occur following being converted into meprobamate.[3]
Carisoprodol was approved for medical use in the United States in 1959.[3] Its approval in the European Union was withdrawn in 2008.[5] It is available as a generic medication.[3] In 2019, it was the 343rd most commonly prescribed medication in the United States, with more than 800 thousand prescriptions.[6] In the United States, it is a Schedule IV controlled substance.[3]
Medical uses
Edit
Carisoprodol is meant to be used along with rest, physical therapy and other measures to relax muscles after strains, sprains and muscle injuries.[7] It comes in tablet format and is taken by the mouth three times a day and before bed.[7]
Side effects
Edit
The usual dose of 350 mg is unlikely to engender prominent side effects other than somnolence, and mild to significant euphoria or dysphoria, but the euphoria is generally short-lived due to the fast metabolism of carisoprodol into meprobamate and other metabolites; the euphoria derived is, according to new research[citation needed], most likely due to carisoprodol’s inherent, potent anxiolytic effects that are far stronger than those produced by its primary metabolite, meprobamate, which is often misblamed for the drug-seeking associated with carisoprodol, as carisoprodol itself is responsible for the significantly more intense central nervous system effects than meprobamate alone. Carisoprodol has a qualitatively different set of effects to that of meprobamate (Miltown). The medication is well tolerated and without adverse effects in the majority of patients for whom it is indicated. In some patients, however, and/or early in therapy, carisoprodol can have the full spectrum of sedative side effects and can impair the patient’s ability to operate a firearm, motor vehicles, and other machinery of various types, especially when taken with medications containing alcohol, in which case an alternative medication would be considered. The intensity of the side effects of carisoprodol tends to lessen as therapy continues, as is the case with many other drugs. Other side effects include: dizziness, clumsiness, headache, fast heart rate, upset stomach, vomiting and skin rash.[7]
The interaction of carisoprodol with essentially all opioids, and other centrally acting analgesics, but especially codeine, those of the codeine-derived subgroup of the semisynthetic class (ethylmorphine, dihydrocodeine, hydrocodone, oxycodone, nicocodeine, benzylmorphine, the various acetylated codeine derivatives including acetyldihydrocodeine, dihydroisocodeine, nicodicodeine and others) which allows the use of a smaller dose of the opioid to have a given effect, is useful in general and especially where skeletal muscle injury and/or spasm is a large part of the problem. The potentiation effect is also useful in other pain situations and is also especially useful with opioids of the open-chain class, such as methadone, levomethadone, ketobemidone, phenadoxone and others. In recreational drug users, deaths have resulted from combining doses of hydrocodone and carisoprodol. Another danger of misuse of carisoprodol and opiates is the potential to asphyxiate while unconscious.[citation needed]
Meprobamate and other muscle-relaxing drugs often were subjects of misuse in the 1950s and 60s.[8][9] Overdose cases were reported as early as 1957, and have been reported on several occasions since then.[10][11][12][13][14][15][16]
Carisoprodol is metabolized by the liver and excreted by the kidneys, so this drug must be used with caution with patients that have impaired hepatic or renal function.[17] Because of potential for more severe side effects, this drug is on the list to avoid for elderly people.[18]
Withdrawal
Edit
Carisoprodol, meprobamate, and related drugs such as tybamate, have the potential to produce physical dependence of the barbiturate type following periods of prolonged use. Withdrawal of the drug after extensive use may require hospitalization in medically compromised patients. In severe cases the withdrawal can mimic the symptoms of alcohol withdrawal including the potentially lethal status epilepticus.
Psychological dependence has also been linked to carisoprodol use[19] although this is much less severe than with meprobamate itself (presumably due to the slower onset of effects). Psychological dependence is more common in those who use carisoprodol non-medically and those who have a history of substance use (particularly sedatives or alcohol). It may reach clinical significance before physiological tolerance and dependence have occurred and (as with benzodiazepines) has been demonstrated to persist to varying degrees of severity for months or years after discontinuation.
Discontinuation of carisoprodol, as with all GABA-ergics, can result in cognitive changes which persist for weeks, months, or rarely even years including greatly increased anxiety and depression, social withdrawal, hair-trigger agitation/aggression, chronic insomnia, new or aggravated (often illogical) phobias, reduced IQ, short term and long-term memory loss, and dozens of other sequelae.[20] The effects, severity, and duration appear to be slightly dose-dependent but are mainly determined by the patients pattern of use (taken as prescribed, taken in bulk doses, mixed with other drugs, a combination of the above, etc.), genetic predisposition to substance use, and a history of substance use all increase the patients risk of persistent discontinuation syndrome symptoms.
Treatment for physical withdrawal generally involves switching the patient to a long-acting benzodiazepine such as diazepam or clonazepam then slowly titrating them off the replacement drug completely at a rate which is both reasonably comfortable for the patient but rapid enough for the managing physician to consider the rate of progress acceptable (overly rapid dose reduction greatly increases the risk of patient non-compliance such as the use of illicitly obtained alternative sedatives and/or alcohol). Psychotherapy and cognitive behavioral therapy have demonstrated moderate success in reducing the rebound anxiety which results upon carisoprodol discontinuation but only when combined with regular and active attendance to a substance use support group.[citation needed]
Carisoprodol withdrawal can be life-threatening (especially in high dose users and those who attempt to quit «cold turkey»). Medical supervision is recommended, with gradual reduction of dose of carisoprodol or a substituted medication, typical of other depressant drugs.
Non-medical use
Edit
Combining a muscle relaxant like carisoprodol with opioids and benzodiazepines is referred to as «The Holy Trinity» as it has been reported to increase the power of the «high».[21]
Recreational users of carisoprodol usually seek its potentially heavy sedating, relaxant, and anxiolytic effects.[22] Also, because of its potentiating effects on narcotics, it is often used in conjunction with many opioid drugs. Also it is not detected on standard drug testing screens. On 26 March 2010 the DEA issued a Notice of Hearing on proposed rule making in respect to the placement of carisoprodol in schedule IV of the Controlled Substances Act.[23] The DEA ended up classifying it under schedule IV.[24] Carisoprodol is sometimes mixed with date rape drugs.[25]
Many overdoses have resulted from recreational users combining these drugs to combine their individual effects without being aware of the enzyme-induction induced potentiation.[medical citation needed]
Overdose
Edit
As with other GABAergic drugs, combination with other GABAergic drugs, including alcohol, as well as with sedatives in general, possess a significant risk to the user in the form of overdose. Overdose symptoms are similar to those of other GABAergics including excessive sedation and unresponsiveness to stimuli, severe ataxia, amnesia, confusion, agitation, intoxication and inappropriate (potentially violent) behavior. Severe overdoses may present with respiratory depression (and subsequent pulmonary aspiration), coma, and death.[citation needed]
Carisoprodol is not detected on all toxicology tests which may delay diagnosis of overdose. Overdose symptoms in combination with opiates are similar but are distinguished by the presentation of normal or pinpoint pupils, which are generally unresponsive to light. Carisoprodol (as with its metabolite meprobamate) is particularly dangerous in combination with alcohol. Flumazenil (the benzodiazepine antidote) is not effective in the management of carisoprodol overdose as carisoprodol acts at the barbiturate binding site. Treatment mirrors that of barbiturate overdoses and is generally supportive, including the administration of mechanical respiration and pressors as implicated (and in rare cases, bemegride). Total amnesia of the experience is not uncommon following recovery.[citation needed]
In 2014 actress Skye McCole Bartusiak died of an overdose due to the combined effects of carisoprodol, hydrocodone and difluoroethane.[26]
Pharmacology
Edit
Pharmacodynamics
Edit
Carisoprodol, has a chemical structure similar to Glutamate, a neurotransmitter, and dimethylglycine. Upon analysis[citation needed], this pharmacological agent seems to be an agonist of the NMDA receptor, with an unknown [Km].
Because excess Glutamate causes excitotoxicity and neuronal apoptosis, Carisoprodol overdose may also lead to NMDA related toxicity, thus inducing seizures at high doses, and muscle relaxation upon administration.
Carisoprodol’s structural similarity to Meprobamate indicates GABAergic activity, including GABA A agonism, similar to the mechanism of benzodiazepines.[27]
This will allow for further muscle relaxation and anxiety reduction. Therefore, Carisoprodol, at low to moderate dosages, may be clinically indicated for absent seizures, yet exacerbate Tonic-clonic seizures.
Pharmacokinetics
Edit
Carisoprodol has a rapid, 30-minute onset of action, with the aforementioned effects lasting about two to six hours. It is metabolized in the liver via the cytochrome P450 oxidase isozyme CYP2C19, excreted by the kidneys and has about an eight-hour half-life. In patients with low levels of CYP2C19 (poor metabolizers), standard doses can lead to increased concentrations of carisoprodol (up-to a four-fold increase).[28] A considerable proportion of carisoprodol is metabolized to meprobamate, which is a known addictive substance; this could account for the addictive potential of carisoprodol (meprobamate levels reach higher peak plasma levels than carisoprodol itself following administration). As mentioned above, carisoprodol appears to have strong anxiolytic effects on its own; however, a large part of its effects also come from the fact that it is metabolized into meprobamate: at least a 25% of the carisoprodol administered will be transformed into meprobamate which means that meprobamate is 3.25× stronger than carisoprodol (although this rate varies from person to person according to their levels of CYP2C19 enzymes in their livers with some people having considerably higher levels) or, in other words, 200 mg of meprobamate (which is the lowest standard dose) is equivalent to 650 mg of carisoprodol.[2] As such, meprobamate is believed to play a significant role in the effects of carisoprodol and meprobamate’s long half-life results in bioaccumulation following extended periods of carisoprodol administration.
It is slightly soluble in water and freely soluble in ethanol, chloroform and acetone. The drug’s solubility is practically independent of pH.
History
Edit
On 1 June 1959, several American pharmacologists convened at Wayne State University in Detroit, Michigan to discuss a newly discovered structural analogue of meprobamate. The substitution of one hydrogen atom with an isopropyl group on one of the carbamyl nitrogens was intended to yield a drug with new pharmacological properties. It had been developed by Frank Berger at Wallace Laboratories and was named carisoprodol.[29]
Building on meprobamate’s pharmacological effects, carisoprodol was intended to have better muscle relaxing properties, less potential for addiction, and a lower risk of overdose. Carisoprodol’s effect profile did indeed turn out to differ significantly with respect to meprobamate, with carisoprodol possessing stronger muscle relaxant and analgesic effects.[30]
Usage and legal status
Edit
Norway
Edit
Reports from Norway have shown carisoprodol has addictive potential[31] as a prodrug of meprobamate and/or potentiator of hydrocodone, oxycodone, codeine, and similar drugs. In May 2008 it was taken off the market in Norway.[32]
European Union
Edit
In the EU, the European Medicines Agency issued a release recommending member states suspend marketing authorization for this product in the treatment of acute (not chronic) back pain.[33]
As of November 2007, carisoprodol has been taken off the market in Sweden due to problems with dependence and side effects. The agency overseeing pharmaceuticals considered other drugs used with the same indications as carisoprodol to have the same or better effects without the risks of the drug.[34]
United States
Edit
Until 12 December 2011, when the administrator of the Drug Enforcement Administration (DEA) issued the final ruling placing the substance carisoprodol into Schedule IV of the Controlled Substances Act (CSA), carisoprodol was not a controlled substance. The placement of carisoprodol into Schedule IV was effective 11 January 2012.[35]
Carisoprodol is available generically as 350 mg and, more recently, 250 mg tablets. Compounded tablets with acetaminophen and codeine are also available.[36]
Canada
Edit
Federally, carisoprodol is a prescription drug (Schedule I, sub-schedule F1).[37] Provincial regulations vary.[38] It is no longer readily available.[medical citation needed]
Indonesia
Edit
- In September 2013, carisoprodol was taken off the market due to problems with diversion, dependence and side effects.
- In September 2017, one child died and 50 had seizures when PCC, which stands for «Paracetamol Caffeine Carisoprodol» was mixed (probably illicit) into children’s drinks in elementary and junior high schools in Kendari.[39]
Notes
Edit
- ^ At least 25% of the carisoprodol in the body is transformed by the liver into meprobamate, its main active metabolite, which in turn has a half-life of 10 hours.[2]
References
Edit
- ^ «Carisoprodol». drugs.com. Retrieved 16 April 2017.
- ^ a b c Carrasco A (13 September 2019). «Letra C (Carisoprodol)». In Carrasco Ruiz MA, Chavez Pulido X, Morales E (eds.). Diccionario de Especialidades Farmaceúticas PLM. Diccion (in Spanish). Vol. I (65th ed.). Mexico City: PLM Latinoamérica. p. 222. ISBN 978-607-625-072-3. Retrieved 13 June 2021.
- ^ a b c d e f g h i j k l m «Carisoprodol Monograph for Professionals». Drugs.com. American Society of Health-System Pharmacists. Retrieved 8 April 2019.
- ^ «DailyMed — carisoprodol tablet». dailymed.nlm.nih.gov. Retrieved 8 April 2019.
- ^ «Carisoprodol». European Medicines Agency. 15 November 2007. Retrieved 8 April 2019.
- ^ «Carisoprodol — Drug Usage Statistics». ClinCalc. Retrieved 7 October 2022.
- ^ a b c «Carisoprodol». MedlinePlus. National Library of Medicine. Retrieved 6 May 2019.
- ^ Kamin I, Shaskan DA (June 1959). «Death due to massive overdose of meprobamate». The American Journal of Psychiatry. 115 (12): 1123–1124. doi:10.1176/ajp.115.12.1123-a. PMID 13649976.
- ^ Hollister LE (1983). «The pre-benzodiazepine era». Journal of Psychoactive Drugs. 15 (1–2): 9–13. doi:10.1080/02791072.1983.10472117. PMID 6350551.
- ^ Gaillard Y, Billault F, Pépin G (May 1997). «Meprobamate overdosage: a continuing problem. Sensitive GC-MS quantitation after solid phase extraction in 19 fatal cases». Forensic Science International. 86 (3): 173–180. doi:10.1016/S0379-0738(97)02128-2. PMID 9180026.
- ^ Allen MD, Greenblatt DJ, Noel BJ (December 1977). «Meprobamate overdosage: a continuing problem». Clinical Toxicology. 11 (5): 501–515. doi:10.3109/15563657708988216. PMID 608316.
- ^ Kintz P, Tracqui A, Mangin P, Lugnier AA (June 1988). «Fatal meprobamate self-poisoning». The American Journal of Forensic Medicine and Pathology. 9 (2): 139–140. doi:10.1097/00000433-198806000-00009. PMID 3381792.
- ^ Eeckhout E, Huyghens L, Loef B, Maes V, Sennesael J (1988). «Meprobamate poisoning, hypotension and the Swan-Ganz catheter». Intensive Care Medicine. 14 (4): 437–438. doi:10.1007/BF00262904. PMID 3403779. S2CID 2784867.
- ^ Lhoste F, Lemaire F, Rapin M (April 1977). «Treatment of hypotension in meprobamate poisoning». The New England Journal of Medicine. 296 (17): 1004. doi:10.1056/NEJM197704282961717. PMID 846530.
- ^ Bedson HS (February 1959). «Coma due to meprobamate intoxication; report of a case confirmed by chemical analysis». Lancet. 1 (7067): 288–290. doi:10.1016/S0140-6736(59)90209-0. PMID 13632000.
- ^ Blumberg AG, Rosett HL, Dobrow A (September 1959). «Severe hypotensive reactions following meprobamate overdosage». Annals of Internal Medicine. 51 (3): 607–612. doi:10.7326/0003-4819-51-3-607. PMID 13801701.
- ^ «CARISOPRODOL». TOXNET. National Library of Medicine. Retrieved 6 May 2019.
- ^ NCQA’s HEDIS Measure: Use of High Risk Medications in the Elderly Archived 1 February 2010 at the Wayback Machine
- ^ «What is Carisoprodol used for?». Pain o Soma medicines. 19 March 2021. Retrieved 29 April 2021.
- ^ Barker MJ, Greenwood KM, Jackson M, Crowe SF (April 2004). «Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: a meta-analysis». Archives of Clinical Neuropsychology. 19 (3): 437–454. doi:10.1016/S0887-6177(03)00096-9. PMID 15033227.
- ^ Horsfall JT, Sprague JE (February 2017). «The Pharmacology and Toxicology of the ‘Holy Trinity’«. Basic & Clinical Pharmacology & Toxicology. 120 (2): 115–119. doi:10.1111/bcpt.12655. PMID 27550152. S2CID 25909460.
- ^ «DEA Drugs & Chemicals of Concern «Carisoprodol»«. Archived from the original on 17 April 2011. Retrieved 29 April 2011.
- ^ «Schedules of Controlled Substances: Placement of Carisoprodol Into Schedule IV; Announcement of Hearing». Archived from the original on 15 July 2011. Retrieved 19 April 2010.
- ^ «Carisoprodol» (PDF). Drug Enforcement Administration, Diversion Control Division, Drug & Chemical Evaluation Section. U.S. Department of Justice. December 2019.
- ^ Madea B, Musshoff F (May 2009). «Knock-out drugs: their prevalence, modes of action, and means of detection». Deutsches Ärzteblatt International. 106 (20): 341–347. doi:10.3238/arztebl.2009.0341. PMC 2689633. PMID 19547737.
- ^ Duke A (22 July 2014). «‘Patriot’ actress Skye McCole Bartusiak dead at 21″. CNN Entertainment. Retrieved 24 February 2019.
- ^ Conermann T, Christian D (2022). «Carisoprodol». StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31971718. Retrieved 23 February 2022.
- ^ Dean L (4 April 2017). Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, Dean L (eds.). «Carisoprodol Therapy and CYP2C19 Genotype». Medical Genetics Summaries. PMID 28520382.
- ^ Miller JG, ed. The pharmacology and clinical usefulness of carisoprodol. Detroit:Wayne State University; 1959.
- ^ Berger FM, Kletzkin M, Ludwig BJ, Margolin S (March 1960). «The history, chemistry, and pharmacology of carisoprodol». Annals of the New York Academy of Sciences. 86 (1): 90–107. Bibcode:1960NYASA..86…90B. doi:10.1111/j.1749-6632.1960.tb42792.x. PMID 13799302. S2CID 11909344.
- ^ Bramness JG, Furu K, Engeland A, Skurtveit S (August 2007). «Carisoprodol use and abuse in Norway: a pharmacoepidemiological study». British Journal of Clinical Pharmacology. 64 (2): 210–218. doi:10.1111/j.1365-2125.2007.02847.x. PMC 2000626. PMID 17298482.
- ^ «Somadril trekkes fra markedet» [Somadril is withdrawn from the market]. Norwegian Medicines Agency (in Norwegian). 20 April 2008. Archived from the original on 16 July 2011. Retrieved 12 March 2010.
- ^ «Carisprodol press release» (PDF). EMEA. Archived from the original (PDF) on 18 July 2009. Retrieved 12 May 2008.
- ^ «Marknadsföringen av Somadril och Somadril comp rekommenderas upphöra tillfälligt» [Marketing of Somadril and Somadril is recommended to cease temporarily] (in Swedish). 16 November 2007. Archived from the original on 23 July 2014. Retrieved 9 May 2009.
- ^ US Department of Justice (2011). «Schedules of Controlled Substances: Placement of Carisoprodol into Schedule IV» (PDF). Federal Register. 76 (238): 77330–77360. Retrieved 1 February 2012.
- ^ «High Cost, No Benefit – The Rheumatologist». the-rheumatologist.org. Archived from the original on 7 May 2015. Retrieved 31 August 2017.
- ^ «NAPRA – Search National Drug Schedule». National Association of Pharmacy Regulatory Authorities. 2009. Archived from the original (ASP) on 1 February 2014. Retrieved 7 January 2014.
- ^ For British Columbia, see library.bcpharmacists.org/D-Legislation_Standards Archived 17 December 2013 at the Wayback Machine
- ^ «One Schoolchild Dies, More Than 50 Suffer Seizures After Consuming Pills in Southeast Sulawesi». Jakarta Globe. 14 September 2017.
Further reading
Edit
- Dean L (2017). «Carisoprodol Therapy and CYP2C19 Genotype». In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520377. Bookshelf ID: NBK425390.
External links
Edit
- «Carisoprodol». Drug Information Portal. U.S. National Library of Medicine.
