(imidacloprid + moxidectin) Topical Solution
Company: Bayer Animal Health
for dogs
Once-a-month topical solution for the prevention of heartworm disease, the treatment of circulating microfilariae, kills adult fleas, is indicated for the treatment of flea infestations, the treatment and control of sarcoptic mange, as well as the treatment and control of intestinal parasite infections in dogs and puppies that are at least 7 weeks of age and that weigh at least 3 lbs.
WARNING
● DO NOT ADMINISTER THIS PRODUCT ORALLY
● For the first 30 minutes after application ensure that dogs cannot lick the product from application sites on themselves or other treated animals.
● Children should not come in contact with application sites for two (2) hours after application.
(See Contraindications, Warnings, Human Warnings, and Adverse Reactions, for more information.)
Advantage Multi (imidacloprid + moxidectin) Topical Solution Caution
Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Advantage Multi® for Dogs (10% imidacloprid + 2.5% moxidectin) is a colorless to yellow ready-to-use solution packaged in single dose applicator tubes for topical treatment of dogs. The formulation and dosage schedule are designed to provide a minimum of 4.5 mg/lb (10 mg/kg) imidacloprid and 1.1 mg/lb (2.5 mg/kg) moxidectin based on body weight.
Imidacloprid is a chloronicotinyl nitroguanidine insecticide. The chemical name for imidacloprid is 1-[(6-Chloro-3-pyridinyl)methyl]-N-nitro-2-imidazolidinimine. Moxidectin is a semisynthetic macrocyclic lactone endectocide derived from the actinomycete Streptomycetes cyaneogriseus noncyanogenus. The chemical name for moxidectin is [6R, 23E, 25S(E)]-5-O- Demethyl-28-deoxy-25-(1,3-dimethyl-1-butenyl)-6,28-epoxy-23-(methoxyimino) milbemycin B.
Advantage Multi (imidacloprid + moxidectin) Topical Solution Indications
Advantage Multi for Dogs is indicated for the prevention of heartworm disease caused by Dirofilaria immitis and the treatment of Dirofilaria immitis circulating microfilariae in heartworm-positive dogs. Advantage Multi for Dogs kills adult fleas and is indicated for the treatment of flea infestations (Ctenocephalides felis). Advantage Multi for Dogs is indicated for the treatment and control of sarcoptic mange caused by Sarcoptes scabiei var. canis. Advantage Multi for Dogs is also indicated for the treatment and control of the following intestinal parasites:
|
Intestinal Parasite |
Intestinal Stage |
|||
|
Adult |
Immature Adult |
Fourth Stage Larvae |
||
|
Hookworm Species |
Ancylostoma caninum |
X |
X |
X |
|
Uncinaria stenocephala |
X |
X |
X |
|
|
Roundworm Species |
Toxocara canis |
X |
X |
|
|
Toxascaris leonina |
X |
|||
|
Whipworm |
Trichuris vulpis |
X |
Contraindications
Do not administer this product orally. (See WARNINGS.)
Do not use this product (containing 2.5% moxidectin) on cats.
Warnings
For the first 30 minutes after application:
Ensure that dogs cannot lick the product from application sites on themselves or other treated dogs, and separate treated dogs from one another and from other pets to reduce the risk of accidental ingestion.
Ingestion of this product by dogs may cause serious adverse reactions including depression, salivation, dilated pupils, incoordination, panting, and generalized muscle tremors.
In avermectin sensitive dogs,a the signs may be more severe and may include coma and death.b
a Some dogs are more sensitive to avermectins due to a mutation in the MDR1 gene. Dogs with this mutation may develop signs of severe avermectin toxicity if they ingest this product. The most common breeds associated with this mutation include Collies and Collie crosses.
b Although there is no specific antagonist for avermectin toxicity, even severely affected dogs have completely recovered from avermectin toxicity with intensive veterinary supportive care.
HUMAN WARNINGS:
Not for human use. Keep out of the reach of children.
Children should not come in contact with application sites for two (2) hours after application.
Causes eye irritation. Harmful if swallowed. Do not get in eyes or on clothing. Avoid contact with skin. Exposure to the product has been reported to cause headache; dizziness; and redness, burning, tingling, or numbness of the skin. Wash hands thoroughly with soap and warm water after handling.
If contact with eyes occurs, hold eyelids open and flush with copious amounts of water for 15 minutes. If eye irritation develops or persists, contact a physician. If swallowed, call poison control center or physician immediately for treatment advice. Have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by the poison control center or physician. People with known hypersensitivity to benzyl alcohol, imidacloprid or moxidectin should administer the product with caution. In case of allergic reaction, contact a physician. If contact with skin or clothing occurs, take off contaminated clothing. Wash skin immediately with plenty of soap and water. Call a poison control center or physician for treatment advice.
The Safety Data Sheet (SDS) provides additional occupational safety information. For a copy of the Safety Data Sheet (SDS) or to report adverse reactions call Bayer Veterinary Services at 1-800-422-9874. For consumer questions call 1-800-255-6826.
Precautions
Do not dispense dose applicator tubes without complete safety and administration information.
Use with caution in sick, debilitated, or underweight animals. The safety of Advantage Multi for Dogs has not been established in breeding, pregnant, or lactating dogs. The safe use of Advantage Multi for Dogs has not been established in puppies and dogs less than 7 weeks of age or less than 3 lbs. body weight.
Prior to administration of Advantage Multi for Dogs, dogs should be tested for existing heartworm infection. At the discretion of the veterinarian, infected dogs should be treated with an adulticide to remove adult heartworms. The safety of Advantage Multi for Dogs has not been evaluated when administered on the same day as an adulticide. Advantage Multi for Dogs is not effective against adult D. immitis. Although the number of circulating microfilariae is substantially reduced in most dogs following treatment with Advantage Multi for Dogs, the microfilaria count in some heartworm-positive dogs may increase or remain unchanged following treatment with Advantage Multi for Dogs alone or in a dosing regimen with melarsomine dihydrochloride.
(See ADVERSE REACTIONS and ANIMAL SAFETY — Safety Study in Heartworm-Positive Dogs.)
Advantage Multi for Dogs has not been evaluated in heartworm-positive dogs with class 4 heartworm disease.
Adverse Reactions
Heartworm-Negative Dogs
Field Studies: Following treatment with Advantage Multi for Dogs or an active control, dog owners reported the following post-treatment reactions:
|
OBSERVATION |
Advantage Multi n=128 |
Active Control n=68 |
|
Pruritus |
19 dogs (14.8%) |
7 dogs (10.3%) |
|
Residue |
9 dogs (7.0%) |
5 dogs (7.4%) |
|
Medicinal Odor |
5 dogs (3.9%) |
None observed |
|
Lethargy |
1 dog (0.8%) |
1 dog (1.5%) |
|
Inappetence |
1 dog (0.8%) |
1 dog (1.5%) |
|
Hyperactivity |
1 dog (0.8%) |
None observed |
During a field study using 61 dogs with pre-existing flea allergy dermatitis, one (1.6%) dog experienced localized pruritus immediately after imidacloprid application, and one investigator noted hyperkeratosis at the application site of one dog (1.6%).
In a field safety and effectiveness study, Advantage Multi for Dogs was administered to 92 client-owned dogs with sarcoptic mange. The dogs ranged in age from 2 months to 12.5 years and ranged in weight from 3 to 231.5 pounds. Adverse reactions in dogs treated with Advantage Multi for Dogs included hematochezia, diarrhea, vomiting, lethargy, inappetence, and pyoderma.
Laboratory Effectiveness Studies: One dog in a laboratory effectiveness study experienced weakness, depression, and unsteadiness between 6 and 9 days after application of Advantage Multi for Dogs. The signs resolved without intervention by day 10 post-application. The signs in this dog may have been related to peak serum levels of moxidectin, which vary between dogs, and occur between 1 and 21 days after application of Advantage Multi for Dogs.
The following clinical observations also occurred in laboratory effectiveness studies following application with Advantage Multi for Dogs and may be directly attributed to the drug or may be secondary to the intestinal parasite burden or other underlying conditions in the dogs: diarrhea, bloody stools, vomiting, anorexia, lethargy, coughing, ocular discharge and nasal discharge. Observations at the application sites included damp, stiff or greasy hair, the appearance of a white deposit on the hair, and mild erythema, which resolved without treatment within 2 to 48 hours.
Heartworm-Positive Dogs
Field Study: A 56-day field safety study was conducted in 214 D. immitis heartworm and microfilariae positive dogs with Class 1, 2 or 3 heartworm disease. All dogs received Advantage Multi for Dogs on Study Days 0 and 28; 108 dogs also received melarsomine dihydrochloride on Study Days — 14, 14, and 15. All dogs were hospitalized for a minimum of 12 hours following each treatment. Effectiveness against circulating D. immitis microfilariae was > 90% at five of six sites; however, one site had an effectiveness of 73.3%. The microfilariae count in some heartworm-positive dogs increased or remained unchanged following treatment with Advantage Multi for Dogs alone or in a dosing regimen with melarsomine dihydrochloride.
Following treatment with Advantage Multi for Dogs alone or in a dosing regimen with melarsomine dihydrochloride, the following adverse reactions were observed:
|
Adverse Reaction |
Dogs Treated with Advantage Multi for Dogs Only n=106 |
Dogs Treated with Advantage Multi for Dogs + Melarsomine n=108 |
|
Cough |
24 (22.6%) |
25 (23.1%) |
|
Lethargy |
14 (13.2%) |
42 (38.9%) |
|
Vomiting |
11 (10.4%) |
18 (16.7) |
|
Diarrhea, including hemorrhagic |
10 (9.4%) |
22 (20.4%) |
|
Inappetence |
7 (6.6%) |
19 (17.6%) |
|
Dyspnea |
6 (5.7%) |
10 (9.3%) |
|
Tachypnea |
1 (< 1%) |
7 (6.5%) |
|
Pulmonary hemorrhage |
0 |
1 (< 1%) |
|
Death |
0 |
3 (2.8%) |
Three dogs treated with Advantage Multi for Dogs in a dosing regimen with melarsomine dihydrochloride died of pulmonary embolism from dead and dying heartworms. One dog, treated with Advantage Multi for Dogs and melarsomine dihydrochloride, experienced pulmonary hemorrhage and responded to supportive medical treatment. Following the first treatment with Advantage Multi for Dogs alone, two dogs experienced adverse reactions (coughing, vomiting, and dyspnea) that required hospitalization. In both groups, there were more adverse reactions to Advantage Multi for Dogs following the first treatment than the second treatment.
To report a suspected adverse reactions, call 1-800-422-9874.
Post-Approval Experience
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events in dogs are listed in decreasing order of reporting frequency: depression/lethargy, vomiting, pruritus, diarrhea, anorexia, hyperactivity, ataxia, trembling, hypersalivation, application site reactions (alopecia, pruritus, lesions, and erythema), seizures, and anaphylaxis/anaphylactic reactions (hives, urticaria, facial swelling, edema of the head).
Serious reactions, including neurologic signs and death have been reported when cats have been exposed (orally and topically) to this product.
In humans, nausea, numbness or tingling of the mouth/lips and throat, ocular and dermal irritation, pruritus, headache, vomiting, diarrhea, depression and dyspnea have been reported following exposure to this product. To report suspected adverse events and/or obtain a copy of the SDS or for technical assistance, call Bayer Animal Heath at 1-800-422-9874.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
Dosage and Administration
The recommended minimum dose is 4.5 mg/lb (10 mg/kg) imidacloprid and 1.1 mg/lb (2.5 mg/kg) moxidectin, once a month, by topical administration.
Do not apply to irritated skin.
1. Remove one dose applicator tube from the package. As specified in the following table, administer the entire contents of the Advantage Multi for Dogs tube that correctly corresponds with the body weight of the dog.
|
Dog (lb.) |
Advantage Multi For Dogs |
Volume (mL) |
Imidacloprid (mg) |
Moxidectin (mg) |
|
3 — 9 |
Advantage Multi 9 |
0.4 |
40 |
10 |
|
9.1 — 20 |
Advantage Multi 20 |
1.0 |
100 |
25 |
|
20.1 — 55 |
Advantage Multi 55 |
2.5 |
250 |
62.5 |
|
55.1 — 88 |
Advantage Multi 88 |
4.0 |
400 |
100 |
|
88.1 — 110* |
Advantage Multi 110 |
5.0 |
500 |
125 |
*Dogs over 110 lbs. should be treated with the appropriate combination of Advantage Multi for Dogs tubes.
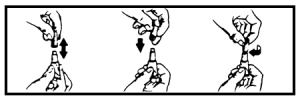
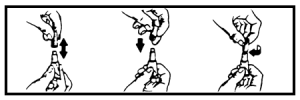
2. While holding the tube in an upright position, remove the cap from the tube.
3. Turn the cap over and push the other end of cap onto the tip of the tube.
4. Twist the cap to break the seal and then remove cap from the tube.
5. The dog should be standing for application. Part the hair on the back of the dog between the shoulder blades until the skin is visible. For dogs weighing 20 lbs. or less, place the tip of the tube on the skin and apply the entire contents directly on the exposed skin at one spot between the shoulder blades. For dogs weighing more than 20 lbs., place the tip of the tube on the skin and apply the entire contents directly on the exposed skin at 3 or 4 spots on the top of the backline from the base of the neck to the upper back in an area inaccessible to licking. Do not apply an amount of solution at any one location that could run off the side of the dog.
Do not let this product get in your dog’s mouth or eyes. Do not allow the dog to lick any of the application sites for 30 minutes. In households with multiple pets, keep each treated dog separated from other treated dogs and other pets for 30 minutes after application to prevent licking the application sites. (See WARNINGS.)
Stiff hair, a damp appearance of the hair, pink skin, or a slight powdery residue may be observed at the application site on some animals. This is temporary and does not affect the safety and effectiveness of the product.
Shampooing 90 minutes after treatment does not reduce the effectiveness of Advantage Multi for Dogs in the prevention of heartworm disease.
Shampooing or water immersion 4 days after treatment will not reduce the effectiveness of Advantage Multi for Dogs in the treatment of flea infestations. However, shampooing as often as once weekly may reduce the effectiveness of the product against fleas.
Heartworm Prevention: For prevention of heartworm disease, Advantage Multi for Dogs should be administered at one-month intervals. Advantage Multi for Dogs may be administered year-round or at a minimum should start one month before the first expected exposure to mosquitoes and should continue at monthly intervals until one month after the last exposure to mosquitoes. If a dose is missed and a 30-day interval between doses is exceeded, administer Advantage Multi for Dogs immediately and resume the monthly dosing schedule. When replacing another heartworm preventative product in a heartworm prevention program, the first treatment with Advantage Multi for Dogs should be given within one month of the last dose of the former medication.
Treatment of Circulating Microfilariae: For the treatment of circulating D. immitis microfilariae in heartworm-positive dogs, Advantage Multi for Dogs should be administered at one-month intervals. Treatment with an approved adulticide therapy is recommended because Advantage Multi for Dogs is not effective for the treatment of adult D. immitis.
(See PRECAUTIONS.)
Flea Treatment: For the treatment of flea infestations, Advantage Multi for Dogs should be administered at one-month intervals. If the dog is already infested with fleas when the first dose of Advantage Multi for Dogs is administered, adult fleas on the dog will be killed. However, reinfestation from the emergence of pre-existing pupae in the environment may continue to occur for six weeks or longer after treatment is initiated. Dogs treated with imidacloprid, including those with pre-existing flea allergy dermatitis have shown clinical improvement as a direct result of elimination of fleas from the dog.
Treatment and Control of Intestinal Nematode Infections:
For the treatment and control of intestinal hookworm infections caused by Ancylostoma caninum and Uncinaria stenocephala (adults, immature adults and fourth stage larvae) and roundworm infections caused by Toxocara canis (adults and fourth stage larvae), and Toxascaris leonina (adults), and whipworm infections caused by Trichuris vulpis (adults), Advantage Multi for Dogs should be administered once as a single topical dose.
Treatment and Control of Sarcoptic Mange: For the treatment and control of sarcoptic mange caused by Sarcoptes scabiei var. canis, Advantage Multi for Dogs should be administered as a single topical dose. A second monthly dose may be administered if necessary.
ANIMAL SAFETY:
Heartworm-Negative Dogs
Field Study: In a controlled, double-masked, field safety study, Advantage Multi for Dogs was administered to 128 dogs of various breeds, 3 months to 15 years of age, weighing 4 to 157 pounds. Advantage Multi for Dogs was used safely in dogs concomitantly receiving ACE inhibitors, anticonvulsants, antihistamines, antimicrobials, chondroprotectants, corticosteroids, immunotherapeutics, MAO inhibitors, NSAIDs, ophthalmic medications, sympathomimetics, synthetic estrogens, thyroid hormones, and urinary acidifiers. Owners reported the following signs in their dogs after application of Advantage Multi for Dogs: pruritus, flaky/greasy residue at the treatment site, medicinal odor, lethargy, inappetence, and hyperactivity. (See ADVERSE REACTIONS.)
Safety Study in Puppies: Advantage Multi for Dogs was applied topically at 1, 3 and 5X the recommended dose to 7-week-old Beagle puppies once every 2 weeks for 6 treatments on days 0, 14, 28, 42, 56, and 70. Loose stools and diarrhea were observed in all groups, including the controls, throughout the study. Vomiting was seen in one puppy from the 1X treatment group (day 57), in two puppies from the 3X treatment group (days 1 and 79), and in one puppy from the 5X treatment group (day 1). Two puppies each in the 1X, 3X, and 5X groups had decreased appetites within 24 hours post-dosing. One puppy in the 1X treatment group had pruritus for one hour following the fifth treatment. A puppy from the 5X treatment group displayed rapid, difficult breathing from 4 to 8 hours following the second treatment.
Dermal Dose Tolerance Study: Advantage Multi for Dogs was administered topically to 8-month-old Beagle dogs at 10X the recommended dose once. One dog showed signs of treatment site irritation after application. Two dogs vomited, one at 6 hours and one at 6 days post-treatment. Increased RBC, hemoglobin, activated partial thromboplastin, and direct bilirubin were observed in the treated group. Dogs in the treated group did not gain as much weight as the control group.
Oral Safety Study in Beagles: Advantage Multi for Dogs was administered once orally at the recommended topical dose to 12 dogs. Six dogs vomited within 1 hour of receiving the test article, 2 of these dogs vomited again at 2 hours, and 1 dog vomited again up to 18 hours post-dosing. One dog exhibited shaking (nervousness) 1 hour post-dosing. Another dog exhibited abnormal neurological signs (circling, ataxia, generalized muscle tremors, and dilated pupils with a slow pupillary light response) starting at 4 hours post-dosing through 18 hours post-dosing. Without treatment, this dog was neurologically normal at 24 hours and had a normal appetite by 48 hours post-dosing.
(See CONTRAINDICATIONS.)
Dermal Safety Study in Ivermectin-Sensitive Collies:
Advantage Multi for Dogs was administered topically at 3 and 5X the recommended dose every 28 days for 3 treatments to Collies which had been pre-screened for avermectin sensitivity. No clinical abnormalities were observed.
Oral Safety Study in Ivermectin-Sensitive Collies:
Advantage Multi for Dogs was administered orally to 5 pre-screened ivermectin-sensitive Collies. The Collies were asymptomatic after ingesting 10% of the minimum labeled dose. At 40% of the minimum recommended topical dose, 4 of the dogs experienced neurological signs indicative of avermectin toxicity including depression, ataxia, mydriasis, salivation, muscle fasciculation, and coma, and were euthanized.
(See CONTRAINDICATIONS.)
Heartworm-Positive Dogs
Laboratory Safety Study in Heartworm-Positive Dogs: Advantage Multi for Dogs was administered topically at 1 and 5X the recommended dose every 14 days for 3 treatments to dogs with adult heartworm infections and circulating microfilariae. At 5X, one dog was observed vomiting three hours after the second treatment. Hypersensitivity reactions were not seen in the 5X treatment group. Microfilariae counts decreased with treatment.
STORAGE INFORMATION:
Store at temperatures between 4°C (39°F) and 25°C (77°F), avoiding excess heat or cold.
How Supplied
Applications Per Package
6 x 0.4 mL tubes
6 x 1.0 mL tubes
6 x 2.5 mL tubes
6 x 4.0 mL tubes
6 x 5.0 mL tubes
for cats
Once-a-month topical solution for cats for the prevention of heartworm disease, kills adult fleas, is indicated for the treatment of flea infestations, as well as the treatment and control of ear mite infestations and intestinal parasite infections in cats and kittens 9 weeks of age and older and that weigh at least 2 lbs.
Once-a-month topical solution for ferrets for the prevention of heartworm disease, kills adult fleas, and is indicated for the treatment of flea infestations. Indicated for ferrets that weigh at least 2 lbs.
Advantage Multi (imidacloprid + moxidectin) Topical Solution Caution
Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Advantage Multi® for Cats (10% imidacloprid + 1% moxidectin) is a colorless to yellow ready-to-use solution packaged in single-dose applicator tubes for topical treatment of cats. The formulation and dosage schedule are designed to provide a minimum of 4.5 mg/lb (10.0 mg/kg) imidacloprid and 0.45 mg/lb (1.0 mg/kg) moxidectin based on body weight.
Imidacloprid is a chloronicotinyl nitroguanidine insecticide. The chemical name of imidacloprid is 1-[(6-Chloro-3-pyridinyl)methyl]-N-nitro-2-imidazolidinimine. Moxidectin is a semisynthetic macrocyclic lactone endectocide derived from the actinomycete Streptomycetes cyaneogriseus noncyanogenus. The chemical name of moxidectin is [6R, 23E, 25S(E)]-5-O- Demethyl-28-deoxy-25-(1,3-dimethyl-1-butenyl)-6,28-epoxy-23-(methoxyimino) milbemycin B.
Advantage Multi (imidacloprid + moxidectin) Topical Solution Indications
Advantage Multi for Cats is indicated for the prevention of heartworm disease caused by Dirofilaria immitis. Advantage Multi for Cats kills adult fleas (Ctenocephalides felis) and is indicated for the treatment of flea infestations. Advantage Multi for Cats is also indicated for the treatment and control of ear mite (Otodectes cynotis) infestations and the following intestinal parasites:
|
Intestinal Parasite |
Intestinal Stage |
|||
|
Adult |
Immature Adult |
Fourth Stage Larvae |
||
|
Hookworm Species |
Ancylostoma tubaeforme |
X |
X |
X |
|
Roundworm Species |
Toxocara cati |
X |
X |
Warnings
Do not use on sick, debilitated, or underweight cats (See ADVERSE REACTIONS).
Do not use on cats less than 9 weeks of age or less than 2 lbs. body weight.
HUMAN WARNINGS:
Not for human use. Keep out of the reach of children.
Children should not come in contact with the application site for 30 minutes after application.
Causes eye irritation. Harmful if swallowed. Do not get in eyes or on clothing. Avoid contact with skin. Exposure to the product has been reported to cause headache; dizziness; and redness, burning, tingling, or numbness of the skin. Wash hands thoroughly with soap and warm water after handling. If contact with eyes occurs, hold eyelids open and flush with copious amounts of water for 15 minutes. If eye irritation develops or persists, contact a physician. If swallowed, call poison control center or physician immediately for treatment advice. Have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by the poison control center or physician. People with known hypersensitivity to benzyl alcohol, imidacloprid, or moxidectin should administer the product with caution. In case of allergic reaction, contact a physician. If contact with skin or clothing occurs, take off contaminated clothing. Wash skin immediately with plenty of soap and water. Call a poison control center or physician for treatment advice.
The Safety Data Sheet (SDS) provides additional occupational safety information. For a copy of the Safety Data Sheet (SDS) or to report adverse reactions call Bayer Veterinary Services at 1-800-422-9874. For consumer questions call 1-800-255-6826.
Precautions
Do not dispense dose applicator tubes without complete safety and administration information.
Avoid oral ingestion. Cats may experience hypersalivation, tremors, vomiting and decreased appetite if Advantage Multi for Cats is inadvertently administered orally or through grooming/licking of the application site.
The safety of Advantage Multi for Cats has not been established in breeding, pregnant, or lactating cats.
The effectiveness of Advantage Multi for Cats against heartworm infections (D. immitis) after bathing has not been evaluated in cats.
Use of this product in geriatric patients with subclinical conditions has not been adequately studied. Several otherwise healthy, thin geriatric cats experienced prolonged lethargy and sleepiness after using this drug.
(See ADVERSE REACTIONS).
Adverse Reactions
Field Study: Following treatment with Advantage Multi for Cats or an active control, cat owners reported the following post-treatment reactions:
|
OBSERVATION |
Advantage Multi n=113 |
Active Control n=30 |
|
Lethargy (protracted sleeping, poorly responsive) |
3 cats* (2.7%) |
None observed |
|
Behavioral changes (e.g., agitated, excessive grooming, hiding, pacing, spinning) |
9 cats (8.0%) |
1 cat (2.6%) |
|
Discomfort (e.g., scratching, rubbing, head-shaking) |
5 cats (4.4%) |
None observed |
|
Hypersalivation (within 1 hour after treatment) |
3 cats (2.7%) |
None observed |
|
Polydipsia |
3 cats (2.7%) |
None observed |
|
Coughing and gagging |
1 cat (0.9%) |
None observed |
*These three cats were from the same household and included one 13-yr-old cat in good health, one 15-yr-old FIV positive cat in good health, and one 15-yr-old, underweight cat in fair health. Lethargy was noted for 24 to 36 hrs after the first treatment only; one cat was unsteady at 48 hrs. These cats were not on other medications.
During another field study, a 16-year-old cat with renal disease slept in the same place without moving for two days following application. (See PRECAUTIONS).
Laboratory Effectiveness Studies: Advantage Multi for Cats was administered at the recommended dose to 215 cats in 20 effectiveness studies. One random-sourced cat exhibited signs consistent with either moxidectin toxicity or viral respiratory disease and died 26 hours after product application; necropsy findings were inconclusive as to the cause of death. A second cat that became ill 3 days after application of Advantage Multi for Cats responded to treatment for respiratory infection and completed the study. A third cat became ill on day 3 and died with signs and lesions attributable to panleukopenia on day 7 after moxidectin application.
Post-Approval Experience: The following adverse events are based on postapproval adverse drug experience reporting. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events in cats are listed in decreasing order of reporting frequency: hypersalivation, depression/lethargy, application site reactions (alopecia, pruritus, lesions, and erythema), decreased appetite, vomiting, hyperactivity, ataxia, trembling, and behavior disorder (hiding). In some cases death has been reported. In humans, ocular and dermal irritation, nausea, numbness or tingling of the mouth and lips, anaphylaxis, pruritus, vomiting, and tongue/taste abnormalities have been reported following exposure to this product. To report suspected adverse events and/or obtain a copy of the SDS or for technical assistance, call Bayer Animal Heath at 1-890-422-9874. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
Dosage and Administration
The recommended minimum dose is 4.5 mg/lb (10.0 mg/kg) imidacloprid and 0.45 mg/lb (1.0 mg/kg) moxidectin, once a month, by topical administration.
Do not apply to irritated skin.
1. Remove one dose applicator tube from the package. As specified in the following table, administer the entire contents of the Advantage Multi® for Cats tube that correctly corresponds with the body weight of the cat.
|
Cat (lbs.) |
Advantage Multi for Cats |
Volume (mL) |
Imidacloprid (mg) |
Moxidectin (mg) |
|
2-5 |
Advantage Multi 5 |
0.23 |
23 |
2.3 |
|
5.1-9 |
Advantage Multi 9 |
0.4 |
40 |
4 |
|
9.1-18* |
Advantage Multi 18 |
0.8 |
80 |
8 |
* Cats over 18 lbs. should be treated with the appropriate combination of Advantage Multi for Cats tubes
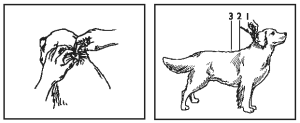
2. While holding the tube in an upright position, remove the cap from the tube.
3. Turn the cap over and push the other end of cap onto the tip of the tube.
4. Twist the cap to break the seal and then remove cap from the tube.

5. Part the hair on the back of the cat’s neck at the base of the head, until the skin is visible. Place the tip of the tube on the skin and apply the entire contents directly on the exposed skin. Lift tube away from skin before releasing pressure on tube.
Do not get this product in the cat’s mouth or eyes or allow the cat to lick the application site for 30 minutes. Treatment at the base of the head will minimize the opportunity for ingestion by grooming. In households with multiple pets, keep animals separated to prevent licking of the application site.
Stiff, matted hair or a damp, oily appearance of the hair may be observed at the application site on some cats. This is temporary and does not affect the safety and effectiveness of the product.
Heartworm Prevention: For prevention of heartworm disease, Advantage Multi for Cats should be administered at one-month intervals. Advantage Multi for Cats may be administered year-around or at a minimum should start one month before the first expected exposure to mosquitoes and should continue at monthly intervals until one month after the last exposure to mosquitoes. If a dose is missed and a 30-day interval between doses is exceeded, administer Advantage Multi for Cats immediately and resume the monthly dosing schedule. When replacing another heartworm preventative product in a heartworm prevention program, the first treatment with Advantage Multi for Cats should be given within one month of the last dose of the former medication. At the discretion of the veterinarian, cats older than 6 months of age may be tested to determine the presence of existing heartworm infection before treatment with Advantage Multi for Cats (See ADVERSE REACTIONS — Foreign Market Experience).
Flea Treatment: For the treatment of flea infestations, Advantage Multi for Cats should be administered at one-month intervals. If the cat is already infested with fleas when the first dose of Advantage Multi for Cats is administered, adult fleas on the cat will be killed. However, re-infestation from the emergence of pre-existing pupae in the environment may continue to occur for six weeks or longer after treatment is initiated. Cats treated with imidacloprid, including those with pre-existing flea allergy dermatitis have shown clinical improvement as a direct result of elimination of fleas from the cat.
Ear Mite Treatment: For the treatment of ear mites (Otodectes cynotis), Advantage Multi for Cats should be administered once as a single topical dose. Monthly use of Advantage Multi for Cats will control any subsequent ear mite infestations.
Intestinal Nematode Treatment: For the treatment and control of intestinal hookworm infections caused by Ancylostoma tubaeforme (adults, immature adults and fourth stage larvae) and roundworm infections caused by Toxocara cati (adults and fourth stage larvae), Advantage Multi for Cats should be administered once as a single topical dose.
ANIMAL SAFETY:
Studies in Kittens: Advantage Multi for Cats was topically applied at 0, 1, 3, and 5X the maximum dose to 48 healthy 9-week-old kittens on days 0, 28, and 56. Lethargy was observed in 1 kitten from the 3X group and 1 from the 5X group on the day after initial treatment; the kitten from the 3X group was also disoriented and ataxic. One kitten from the 3X group had a slow pupillary light response two days after treatment and one had tremors the day after treatment. Hypersalivation was seen in one kitten from the 5X group approximately six hours post-treatment. One kitten from the 3X group was scratching at the treatment site 2 days after treatment. Slight cough was noted in 7 different kittens (2-0X, 2-1X, and 3-5X) during the 13-day period following the first treatment. Histopathology showed granulomatous inflammation at the treatment site in three 1X kittens. Causal relationship to the drug could not be determined. Pulmonary inflammation (1-5X) and lymphoid hyperplasia (2-1X, 4-3X) were seen in treated kittens. In a second study, Advantage Multi for Cats was topically applied at 0, 1.7, 5.2 and 8.7X the maximum dose to 48 healthy 9-week-old kittens every two weeks for 6 doses. One kitten in the 8.7X group apparently ingested an unknown amount of the drug and developed the following clinical signs prior to euthanasia: mydriasis, salivation, depression, vomiting, unsteadiness, rapid to slow to difficult breathing, poor pupillary response, generalized tremors, inability to move, and nystagmus. Two kittens in the 5.2X group developed mydriasis, salivation, depression, squinting, and poor appetite. A kitten in the 1.7X group developed mydriasis.
Dose Tolerance Study: Eight healthy juvenile cats were topically dosed with a single application of Advantage Multi for Cats at 10 times the recommended dose volume. Mild, transient hypersalivation occurred in two of the cats.
Oral Study in Cats: The oral safety of Advantage Multi for Cats was tested in case of accidental oral ingestion. The maximum topical dose was orally administered to twelve healthy 9-week-old kittens. Hypersalivation (8 of 12 kittens) and vomiting (12 of 12 kittens) were observed immediately post-treatment. Tremors developed in one kitten within 1 hour, resolving without treatment within the next hour. All 12 kittens were either anorexic or had decreased appetite for at least 1 day following treatment. In 3 kittens, the anorexia or decreased appetite continued into the second week following treatment. There was a post-treatment loss of body weight in treated kittens compared to control kittens. In a pilot safety study using kittens younger in age and lighter in weight than allowed by product labeling, an 8-week old kitten weighing 0.6 kg orally received 2X of the label topical dose (0.46 mL/kg). Immediately after dosing, it vomited, had labored breathing and slight tremors. Within 4 hours, it was normal, but was found dead on day 6. Necropsy could not determine the cause of death.
Study in Heartworm Positive Cats: Young adult cats were inoculated subcutaneously with third-stage D. immitis larvae. At 243-245 days post-infection, immunoserology and echocardiography were performed to identify cats with adult heartworm burdens similar to naturally-acquired infections. Two groups were treated topically with either Advantage Multi for Cats at the label dose or placebo, once every 28 days, for three consecutive treatments. A third group was treated topically, once, with Advantage Multi for Cats at 5X the label dose. Sporadic vomiting and labored breathing related to heartworm burden were observed in the treatment and control groups. There was no difference between treatment groups in the numbers of adult D. immitis recovered at study conclusion. No adverse reactions were associated with the topical application of Advantage Multi for Cats to experimentally heartworm-infected cats.
Ferrets
Use only the 0.4 mL Advantage Multi for Cats in ferrets. The 0.23 mL size does not provide an effective dose and the 0.8 mL size could result in an overdose.
Advantage Multi (imidacloprid + moxidectin) Topical Solution Indications
For Ferrets:
Advantage Multi for Cats is indicated for the prevention of heartworm disease in ferrets caused by Dirofilaria immitis. Advantage Multi for Cats kills adult fleas (Ctenocephalides felis) and is indicated for the treatment of flea infestations on ferrets.
Warnings
Do not use on sick or debilitated ferrets.
Precautions
Do not dispense dose applicator tubes without complete safety and administration information.
The safety of Advantage Multi for Cats has not been established in breeding, pregnant, and lactating ferrets.
Treatment of ferrets weighing less than 2.0 lbs (0.9 kg) should be based on a risk-benefit assessment.
The effectiveness of Advantage Multi for Cats in ferrets weighing over 4.4 lbs. (2.0 kg) has not been established.
Adverse Reactions
Field safety Study in Ferrets: Advantage Multi for Cats was topically administered to 131 client-owned ferrets at the recommended dose volume (0.4 mL). The ferrets ranged in age from 3 months to 7 years, and weighed between 0.5 and 1.86 kg (1.1 to 4.1 lbs). The dose of imidacloprid ranged between 21.5 and 80.2 mg/kg in this study. The dose of moxidectin ranged between 2.2 to 8.0 mg/kg in this study.
Adverse reactions in ferrets following treatment included: pruritus/scratching, scabbing, redness, wounds and inflammation at the treatment site; lethargy; and chemical odor. These adverse reactions resolved without additional therapy. Owners also reported stiffening of the hair at the treatment site, however, this is expected with application of a topical product and is not considered an adverse reaction.
Three human adverse reactions were reported. An owner’s finger became red following skin contact with the product. One owner reported a headache caused by chemical odor of the product. One owner reported a tingling sensation of the lips after kissing the treatment site.
Foreign Market Experience: Because the following events were reported voluntarily during post-approval use of the product in foreign markets, it is not always possible to reliably establish a causal relationship to drug exposure. Adverse events reported in ferrets topically treated with 0.4 mL imidacloprid + moxidectin for cats included: malaise, vomiting, diarrhea, shaking, mydriasis, hypersalivation with abnormal neurologic signs, seizures, death, generalized hematoma of the body, and alopecia at the treatment site. Adverse reactions in humans included: burning, tingling, numbness, bad taste in the mouth, dizziness, and headache.
ANIMAL SAFETY:
Ferrets: Advantage Multi for Cats was topically applied at 5X the recommended dose volume to six healthy 9-month-old ferrets on Study Days 0, 14, 28, and 42. Because the weights of the ferrets in this study ranged from 2.0 to 4.0 lb (0.9 kg to 1.8 kg), ferrets received a range of doses from 51.0 to 106.9 mg/lb (112 to 235 mg/kg) of imidacloprid and 5 to 10.5 mg/lb (11 to 23 mg/kg) of moxidectin. The following abnormal clinical signs were reported during the study: wet, matted, and/or greasy appearance to the hair, shaking of the head and/or body, rubbing of dose site on cage, and shedding. Slight increases in phosphorous, potassium, aspartate aminotransferase (AST), and glucose were seen during the study, however, no clinical signs related to these bloodwork changes were reported.
Oral Safety Study: Advantage Multi for Cats was orally administered at the recommended dose volume (0.4 mL) to eight healthy ferrets on Study Day 0. Ferrets were 78 to 101 days old (11.1 to 14.4 weeks) and weighed between 1.1 to 1.8 lb (0.5 to 0.8 kg) body weight on the day of dosing, resulting in doses ranges 22.0-36.8 mg/lb (48.3-81.0 mg/kg) imidacloprid and 2.2-3.7 mg/lb (4.8-8.0 mg/kg) moxidectin. The following abnormal clinical signs were reported immediately following oral administration of Advantage Multi for Cats: vomiting (one ferret) and ataxia (two ferrets). All abnormalities resolved without treatment or supportive care.
Dosage and Administration
For Ferrets:
The recommended minimum dose for a ferret is 9 mg/lb (20.0 mg/kg) imidacloprid and 0.9 mg/lb (2 mg/kg) moxidectin, once a month, by topical administration.
|
Ferret (lbs.) |
Advantage Multi for Cats |
Volume (mL) |
Imidacloprid (mg) |
Moxidectin (mg) |
|
2.0 — 4.4 |
Advantage Multi 9 |
0.4 |
40 |
4 |
Only the 0.4 mL applicator tube volume (Advantage Multi 9) should be used on ferrets.
Do not apply to irritated skin.
1. Remove one dose applicator tube from the package. Administer the entire contents of the Advantage Multi for Cats tube (0.4 mL).
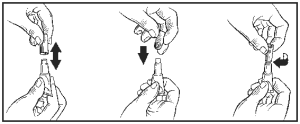
2. While holding the tube in an upright position, remove the cap from the tube.
3. Turn the cap over and push the other end of cap onto the tip of the tube.
4. Twist the cap to break the seal and then remove cap from the tube.

5. Part the hair on the back of the ferret’s neck at the base of the head, until the skin is visible. Place the tip of the tube on the skin and apply the entire contents directly on the exposed skin. Lift tube away from skin before releasing pressure on tube.
Do not get this product in the ferret’s mouth or eyes or allow the ferret to lick the application site for 30 minutes. Treatment at the base of the head will minimize the opportunity for ingestion by grooming. In households with multiple pets, keep animals separated to prevent licking of the application site.
Stiff, matted hair or a damp, oily appearance of the hair may be observed at the application site on some ferrets. This is temporary and does not affect the safety and effectiveness of the product.
Heartworm Prevention: For prevention of heartworm disease, Advantage Multi for Cats should be administered at one-month intervals. Advantage Multi for Cats may be administered year-around or at a minimum should start one month before the first expected exposure to mosquitoes and should continue at monthly intervals until one month after the last exposure to mosquitoes. If a dose is missed and a 30-day interval between doses is exceeded, administer Advantage Multi for Cats immediately and resume the monthly dosing schedule.
Flea Treatment: For the treatment of flea infestations on ferrets, Advantage Multi for Cats should be administered at one-month intervals. If the ferret is already infested with fleas when the first dose of Advantage Multi for Cats is administered, adult fleas on the ferret will be killed. However, re-infestation from the emergence of pre-existing pupae in the environment may continue to occur for six weeks or longer after treatment is initiated.
STORAGE INFORMATION:
Store at temperatures between 4°C (39°F) and 25°C (77°F), avoiding excess heat or cold.
How Supplied
Applications Per Package
3 x 0.23 mL tubes
6 x 0.4 mL tubes
6 x 0.8 mL tubes
Advantage Multi is protected by one or more of the following U.S. patents: 6,232,328, and 6,001,858.
V-03/2016
NADA #141-251, Approved by FDA
Made in Germany
© 2015 Bayer
Bayer, the Bayer Cross and Advantage Multi are registered trademarks of Bayer.
Bayer HealthCare LLC, Animal Health Division, P.O. Box 390, Shawnee Mission, Kansas 66201 U.S.A.
Regulations for product use are established by country. Information contained on this site pertains only to the United States of America, and is not intended to provide adequate information for product use. Before using or dispensing any product, read and carefully observe the label directions.
BAYER HEALTHCARE LLC
Distributed by ELANCO US, INC.
2500 INNOVATION WAY, GREENFIELD, IN, 46140
Copyright © 2023 Animalytix LLC. Updated: 2023-05-29
📜 Инструкция по применению ЦИДЕКТИН 10% LA
💊 Состав препарата ЦИДЕКТИН 10% LA
✅ Применение препарата ЦИДЕКТИН 10% LA
📅 Условия хранения ЦИДЕКТИН 10% LA
⏳ Срок годности ЦИДЕКТИН 10% LA
Описание лекарственного препарата ветеринарного назначения ЦИДЕКТИН 10% LA
Основано на данных Государственного реестра лекарственных средств для ветеринарного применения и сделано в 2018 году
Дата обновления: 2018.04.02
Лекарственная форма
|
|
ЦИДЕКТИН 10% LA |
Раствор для инъекций рег. 840-3-13.13-2545№ПВИ-3-13.13/04043 |
Форма выпуска, состав и упаковка
Международное непатентованное или химическое наименование:
моксидектин
Разработчик:
«Zoetis Inc.», 100 Campus Drive, Florham Park, New Jersey, 07932, США
Производитель:
«Zoetis Manufacturing & Research Spain, S.L.», Ctra. De Camprodon, s/n., Finca La Riba, Vall de Bianya, 17813 Gerona, Испания
Лекарственная форма:
раствор для инъекций
Качественный состав и количественный состав действующих веществ и качественный состав вспомогательных веществ:
моксидектин; бензиловый спирт, вода для инъекций, Миглиол 840=
Количество в потребительской упаковке:
по 50, 200 мл во флаконах
Показания к применению препарата ЦИДЕКТИН 10% LA
Для лечения и профилактики нематодозов и арахноэнтомозов у крупного рогатого скота
Противопоказания к применению препарата ЦИДЕКТИН 10% LA
При индивидуальной чувствительности к компонентам препарата, животным с массой тела менее 100 кг или более 500 кг
Условия хранения ЦИДЕКТИН 10% LA
В закрытой упаковке производителя, в сухом, защищённом от прямых солнечных лучей месте, отдельно от продуктов питания и кормов при температуре от 5 до 25° С
ЦИДЕКТИН 10% LA отзывы
Помогите другим с выбором, оставьте отзыв об ЦИДЕКТИН 10% LA
Оставить отзыв
Если вы хотите разместить ссылку на описание этого препарата — используйте данный код
Моксидектин
Moxidectin
Фармакологическое действие
Моксидектин — антигельминтный препарат, полусинтетическое производное метоксина немадектина, представляющее собой 16-членный пентациклический лактон класса милбемицина. Активен против микрофилярий Onchocerca volvulus, не эффективен для уничтожения взрослых червей. Моксидектин избирательно связывается с GABA-A и глутамат-управляемыми хлорид-ионными каналами паразита, которые жизненно необходимы для функционирования нервных и мышечных клеток беспозвоночных.
Показания
Онхоцеркоз, вызванный Onchocerca volvulus, у пациентов ≥12 лет.
Способ применения и дозы
Перорально: 8 мг в виде разовой дозы.
Взаимодействие
Клинически значимых взаимодействий с другими лекарственными средствами не описано.
Классификация
-
АТХ
P02CX03
-
Фармакологическая группа
-
Код МКБ 10
Информация о действующем веществе Моксидектин предназначена для медицинских и фармацевтических специалистов, исключительно в справочных целях. Инструкция не предназначена для замены профессиональной медицинской консультации, диагностики или лечения. Содержащаяся здесь информация может меняться с течением времени. Наиболее точные сведения о применении препаратов, содержащих активное вещество Моксидектин, содержатся в инструкции производителя, прилагаемой к упаковке.
СТАЙЛАБ предлагает тест-системы для определения моксидектина в моче методом ИФА, а также стандарты моксидектина.
| Иммуноферментный метод анализа (ИФА), стрипованный планшет | 5141MOXI Moxidectin ELISA |
| Стандарты и стандартные растворы | LCS-6198 стандарт моксидектина SPEX |
Моксидектин – это вещество, относящееся к макроциклическим лактонам, или макролидам. Он входит в состав таких средств, как «Гельмимакс», «Адвокат» и других противопаразитарных средств, применяемых в животноводстве и ветеринарии. Моксидектин токсичен для нематод, членистоногих и клещей, поэтому его используют для профилактики и лечения гельминтозов, чесоток и заражения блохами. В зависимости от лекарства и дозировки моксидектин применяют для собак, кошек, птиц, а также лошадей, крупного рогатого скота и овец. В медицине это вещество используют для лечения онхоцеркоза, или речной слепоты – паразитического заболевания, поражающего как животных, так и человека.
Моксидектин хорошо всасывается как при приеме внутрь, так и через кожу. Механизм его действия основан на блокаде ГАМК-зависимых и глутамин-зависимых ионных каналов, что вызывает у беспозвоночных паралич. Этим же обусловлена токсичность моксидектина для водных животных как при разовом, так и при постоянном воздействии. Это вещество накапливается в организме и выводится из него до 30 суток. При длительном употреблении моксидексин нейротоксичен для млекопитающих и птиц. Это проявляется как нарушения чувства равновесия и сонливость, а при тяжелых отравлениях – как атаксия и тремор или кома.
В странах Евросоюза установлены требования к максимально допустимым уровням моксидектина для печени, почек и жировой ткани животных. Согласно «Единым санитарно-эпидемиологическим и гигиеническим требованиям к товарам, подлежащим санитарно-гигиеническому надзору (контролю)», которые действуют в России и странах Таможенного Союза, содержание ветпрепаратов в пищевой продукции животного происхождения необходимо контролировать. Для анализа моксидектина в моче животных удобно использовать тест-системы для проведения ИФА. Они позволяют быстро получить результат, не требуют специального оборудования и просты в работе.
Литература
- Rami Cobb and Albert Boeckh. Moxidectin: a review of chemistry, pharmacokinetics and use in horses. Parasit Vectors. 2009; 2(Suppl 2): S5.
- Prichard R, Ménez C, Lespine A. Moxidectin and the avermectins: Consanguinity but not identity. Int J Parasitol Drugs Drug Resist. 2012 Apr 14;2:134-53.
- Ménez C, Sutra JF, Prichard R, Lespine A. Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab (-/-) mice and effects on mammalian GABA(A) channel activity. PLoS Negl Trop Dis. 2012;6(11):e1883.
ОБЩИЕ СВЕДЕНИЯ
1. Наименование лекарственного препарата для ветеринарного применения:
- торговое наименование: Гельминтал К (Gelmintal К);
- международные непатентованные наименования действующих веществ: моксидектин, празиквантел.
2. Лекарственная форма: раствор для наружного применения.
Гельминтал К в 1 мл в качестве действующих веществ содержит моксидектин — 1,0% и празиквантел — 4,0%, а также вспомогательные вещества: спирт изопропиловый, полиэтиленгликоль и диметилсульфоксид.
3. Гельминтал К по внешнему виду представляет собой прозрачную от бесцветного до светло-желтого цвета маслянистую жидкость.
Срок годности препарата при соблюдении условий хранения — 3 года со дня производства.
Запрещается применение препарата по истечении срока годности.
4. Выпускают Гельминтал К расфасованным в полимерные пипетки по 0,4 мл (для кошек массой менее 4 кг); по 1,0 мл (для кошек массой от 4 до 10 кг).
Пипетки, упакованные по 1 штуке в полимерные блистеры или по 3 штуки, уложенные в картонный вкладыш, помещают по 1 блистеру или по 1 вкладышу вместе с инструкцией по применению в картонные пачки.
5. Хранят препарат в закрытой упаковке производителя, в защищенном от прямых солнечных лучей месте, отдельно от продуктов питания и кормов при температуре от 0 °C до 30 °C и относительной влажности воздуха не более 60%.
6. Гельминтал К следует хранить в местах, недоступных для детей.
7. Неиспользованный препарат утилизируют в соответствии с требованиями законодательства.
8. Условия отпуска: без рецепта ветеринарного врача.
ФАРМАКОЛОГИЧЕСКИЕ СВОЙСТВА
9. Гельминтал К относится к фармакотерапевтической группе: комбинированные противопаразитарные лекарственные препараты.
10. Комбинация моксидектина и празиквантела, входящих в состав препарата, обеспечивает широкий спектр его противопаразитарного действия, в том числе против личиночных и половозрелых фаз развития кишечных нематод, включая Ancylostoma tubaeforme и Toxocara cati, цестод (Dipylidium caninum, Taenia spp., Echinococcus multilocularis), личиночных фаз развития (микрофилярий) Dirofilaria immitis и Dirofilaria repens, демодекозных (Demodex canis) и саркоптоидных (Notoedres cati. Otodectes cynotis) клещей, блох (Stenocephalides canis) и власоедов (Trichodectes canis), паразитирующих у кошек.
Моксидектин — полусинтетическое соединение группы милбемицинов (макроциклические лактоны). Активен в отношении личинок и имаго нематод, насекомых и саркоптоидных клещей.
Оказывает стимулирующее действие на выделение гамма-аминомасляной кислоты, повышает проницаемость мембран для ионов хлора, что подавляет электрическую активность нервных клеток, вызывая нарушение мышечной иннервации, паралич и гибель нематод и эктопаразитов.
Празиквантел — соединение группы пиразинизохинолинов, обладает выраженным действием против половозрелых и неполовозрелых цестод; механизм его действия основан на индуцировании распада тегумента и ингибировании фумаратредуктазы, стойкой деполяризации мышечных клеток гельминта, нарушении энергетического обмена, что вызывает паралич и гибель цестод и способствует их выведению из желудочно-кишечного тракта.
Максимальные концентрации празиквантела и моксидектина при наружном применении отмечаются в крови кошек через 2-4 суток; выводятся соединения из организма в основном с мочой в неизмененном виде и частично в метаболизированной форме в течение 28-30 суток.
Гельминтал К по степени воздействия на организм в соответствии с ГОСТ 12.1.007-76 при пероральном введении относится к умеренно опасным веществам (3 класс опасности), при накожном нанесении — к малоопасным веществам (4 класс опасности), обладает слабо выраженными кумулятивными свойствами; в рекомендуемых дозах не оказывает местно-раздражающего, эмбриотоксического, тератогенного и мутагенного действия.
Препарат хорошо переносится кошками разного возраста и пород; токсичен для пчел, а также рыб и других гидробионтов.
ПОРЯДОК ПРИМЕНЕНИЯ ВЕТЕРИНАРНОГО ПРЕПАРАТА
11. Гельминтал К назначают кошкам с лечебной и профилактической целью при кишечных нематодозах (токсокароз, токсаскаридоз, унцинариоз, анкилостомоз), цестодозах (тениидозы, дипилидиоз, эхинококкозы), энтомозах, вызванных блохами и власоедами, демодекозе, отодектозе и нотоэдрозе, а также для профилактики дирофиляриоза.
12. Противопоказанием к применению Гельминтал К является индивидуальная повышенная чувствительность животного к компонентам препарата, в том числе в анамнезе, и выраженные нарушения функции почек и печени.
Не подлежат обработке истощенные и больные инфекционными болезнями кошки.
Обработку животных массой менее 1 кг при необходимости следует проводить после консультации с ветеринарным врачом.
13. При работе с Гельминталом К следует соблюдать общие правила личной гигиены и техники безопасности, предусмотренные при работе с лекарственными средствами.
Во время работы с препаратом запрещается курить, пить и принимать пищу.
По окончании работы следует тщательно вымыть руки теплой водой с мылом.
Не следует гладить места нанесения препарата и подпускать животное к маленьким детям в течение 24 часов после обработки Гельминталом К.
Запрещается использовать пустые пипетки и картонные пачки из-под лекарственного препарата для бытовых целей.
Их помещают в полиэтиленовый пакет и утилизируют с бытовыми отходами.
При случайном контакте лекарственного препарата с кожей или слизистыми оболочками глаз, их необходимо промыть большим количеством воды.
Людям с гиперчувствительностью к компонентам препарата следует избегать прямого контакта с Гельминталом К.
В случае появления аллергических реакций или при случайном попадании препарата в организм человека следует немедленно обратиться в медицинское учреждение (при себе иметь инструкцию по применению препарата или этикетку).
14. Не подлежат обработке котята моложе 7-недельного возраста, а также беременные и лактирующие кошки.
15. Препарат применяют кошкам путем капельного («spot-оn») нанесения на сухую неповрежденную кожу.
Перед применением препарата кончик пипетки отламывают или отрезают и, раздвинув шерсть, наносят животному на кожу в места, недоступные для слизывания — в область шеи у основания черепа, при обработке крупных животных содержимое пипеток наносят на кожу в 3-4 точки.
Минимальная терапевтическая доза препарата для кошек составляет 0,1 мл/кг массы животного (4 мг/кг празиквантела и 1 мг/кг моксидектина).
В зависимости от массы животного используют препарат Гельминтал К раз личной фасовки в дозах, указанных в таблице:
Таблица
| Масса животного | Маркировка пипеток | Доза препарата (номинальный объем пипетки), количество, штук мл | Доза действующих веществ на 1 кг массы животного, мг | |
| празиквантел | моксидектин | |||
| до 4 кг | для кошек | 0,4 | 4,0 | 1,0 |
| от 4 до 10 кг | для кошек | 1,0 | 10,0 | 2,5 |
При обработке кошек массой более 10 кг препарат применяют в дозе 0,1 мл на каждый кг массы животного.
Для уничтожения блох и вшей обработку животных проводят однократно, для предотвращения повторной инфестации — один раз в 4-6 недель на протяжении всего сезона активности насекомых.
Для лечения отодектоза (ушной чесотки) Гельминтал К применяют накожно однократно.
В процессе лечения рекомендуется очищать слуховой проход от экссудата и струпьев, а в случае осложнения отитом, назначать противомикробные и противовоспалительные средства.
При необходимости курс лечения повторяют через 1 месяц.
С лечебной целью при нотоэдрозе препарат применяют 2-кратно, при демодекозе — 2-4-кратно с интервалом 28 дней; в целях профилактики возможной инвазии — 1 раз месяц.
Лечение демодекоза рекомендуется проводить комплексно с применением этиотропных, патогенетических и симптоматических лекарственных средств.
Для дегельминтизации кошек при нематодозах и цестодозах желудочно- кишечного тракта препарат применяют с лечебной целью однократно, с профилак тической — один раз в месяц.
С целью профилактики дирофиляриоза в неблагополучных по заболеванию регионах препарат применяют в весенне-летне-осенний период: перед началом лета комаров и москитов (переносчиков возбудителя D. Immitis или D. repens) однократно, затем один раз в месяц и последний раз в сезоне не ранее, чем за 1 месяц после завершения лета насекомых.
Гельминтал К не уничтожает половозрелых дирофилярий, но снижает количество циркулирующих в крови микрофилярий, и может быть применен без опасений также и инвазированным животным.
Препарат не следует наносить на влажную или поврежденную кожу, а также мыть животное в течение 4 суток после обработки препаратом.
16. Побочных явлений и осложнений при применении Гельминтал К в соответствии с настоящей инструкцией, как правило, не наблюдается.
В редких случаях возможны индивидуальные реакции кожи (покраснение, зуд), которые самопроизвольно проходят и не требуют применения лекарственных средств.
В случае проявления аллергических реакций у чувствительного к компонентам препарата животного препарат следует тщательно смыть водой с мылом и ополоснуть шерсть большим количеством проточной воды, а при необходимости назначить антигистаминные и симптоматические средства.
17. При передозировке препарата у животного может наблюдаться слабость, тремор, саливация.
Специфические средства детоксикации отсутствуют, применяют общие меры, направленные на выведение лекарственного препарата из организма.
18. Не следует применять Гельминтал К совместно с препаратами, содержащими макроциклические лактоны, и другими противопаразитарными средствами для обработки животных.
19. Особенностей действия лекарственного препарата при его первом применении или его отмене не выявлено.
20. При проведении дегельминтизации следует придерживаться рекомендуемых инструкцией сроков.
В случае пропуска очередной обработки применение препарата возобновляют в той же дозировке по той же схеме.
21. Гельминтал К не предназначен для применения продуктивным животным.
ПОЛНОЕ НАИМЕНОВАНИЕ ИЗГОТОВИТЕЛЯ
АО «Научно-производственная фирма «Экопром»; Московская обл., г. Люберцы, р.п. Томилино, ул. Гаршина, д. 11/23.
