Нитазоксанид
Nitazoxanide
Фармакологическое действие
Нитазоксанид — антипаразитарный и антивирусный препарат широкого спектра действия, используется для лечения различных глистных, протозойных и вирусных инфекций. Показан для лечения инфекции Cryptosporidium parvum и Giardia lamblia у иммунокомпетентных пациентов, для лечения гриппа. Нитазоксанид имеет in vitro противопаразитарную активность и эффективность клинического лечения инфекций, вызванных другими простейшими и гельминтами (например, Entamoeba histolytica, Hymenolepis nana, Ascaris lumbricoides, Cyclospora cayetanensis). Имеются сведения о том, что нитазоксанид обладает эффективностью в лечении ряда вирусных инфекций.
Фармакокинетика
Нитазоксанид быстро метаболизируется до активного метаболита тизоксанида. Исходной реакцией в метаболическом пути нитазоксанида является гидролиз до тизоксанида с последующей конъюгацией, главным образом глюкуронидированием до глюкоронида тизоксанида. In vitro нитазоксанид и тизоксанид подавляют рост спорозоитов и ооцист Cryptosporidium parvum и трофозоитов Giardia lamblia.
Связь тизоксанида с белками плазмы свыше 99 %. Период полувыведения тизоксанида (T½) составляет — 1–1,6 часа. Элиминация с мочой — ~33 %, с фекалиями — ~67 %.
Показания
Лечение диареи у взрослых и детей, вызванной простейшими Giardia lamblia, Cryptosporidium parvum.
Противопоказания
Повышенная чувствительность к нитазоксаниду.
С осторожностью
Печёночная/почечная недостаточность.
Беременность и грудное вскармливание
Применение при беременности
Категория действия на плод по FDA — B.
Адекватных и строго контролируемых исследований по безопасности применения препарата при беременности не проведено.
В исследованиях проведённых на животных (крысы и кролики, при воздействии доз превышающих максимально рекомендованную для человека в 30 и в 2 раза, соответственно) не наблюдалось тератогенности или фетотоксичности.
Применение в период грудного вскармливания
Специальных исследований по безопасности применения препарата в период грудного вскармливания не проведено.
Следует взвесить соотношение польза/риск для матери и ребёнка.
Применение при нарушениях функции печени
При печёночной недостаточности — назначение с осторожностью.
Применение при нарушениях функции почек
При почечной недостаточности — назначение с осторожностью.
Применение в детском возрасте
Безопасность и эффективность суспензии у детей младше 1 года не установлены.
Безопасность и эффективность таблеток у детей младше 12 лет не установлены.
Способ применения и дозы
Перорально (внутрь).
Взрослые
Таблетки 500 мг каждые 12 ч × 3 дня.
Дети
Суспензия:
1–3 года: 5 мл (100 мг) каждые 12 ч × 3 дня.
4–11 лет: 10 мл (200 мг) каждые 12 ч × 3 дня.
Таблетки:
≥12 лет: 500 мг 500 мг каждые 12 ч × 3 дня.
Побочные действия
Боль в животе, головная боль, головокружение, расстройства желудка, рвота, обесцвеченная моча, чрезмерное мочеиспускание, кожная сыпь, зуд, лихорадка, гриппоподобный синдром.
Взаимодействие
Рекомендуется избегать одновременного применения варфарина.
Влияние на способность к вождению автотранспорта и управлению механизмами
При возникновение головокружения необходимо воздержаться от управления транспортными средствами и занятий видами деятельности, требующими повышенной концентрации внимания и быстроты психомоторных реакций.
Классификация
-
АТХ
P01AX11
-
Фармакологическая группа
-
Категория при беременности по FDA
B
(нет риска в исследованиях, не связанных с человеком)
Информация о действующем веществе Нитазоксанид предназначена для медицинских и фармацевтических специалистов, исключительно в справочных целях. Инструкция не предназначена для замены профессиональной медицинской консультации, диагностики или лечения. Содержащаяся здесь информация может меняться с течением времени. Наиболее точные сведения о применении препаратов, содержащих активное вещество Нитазоксанид, содержатся в инструкции производителя, прилагаемой к упаковке.
Химическое название
5-Хлор-N-(2-хлор-4-нитрофенил)-2-гидроксибензамид
Химические свойства
Никлозамид (Niclosamide) — порошок светло-серого или светло-желтого цвета, не имеющий специфического запаха и вкуса, который практически на растворяется в воде.
Вещество открыли в 1958 году и на данный момент оно входит в перечень основных лекарственных средств Всемирной организации здравоохранения.
Вещество нельзя путать с солью никлозамид этаноламина.
Фармакологическое действие
Антигельминтное.
Фармакодинамика и фармакокинетика
Механизм действия заключается в том, что оно парализует паразитов — ленточных гельминтов, снижает степень их устойчивости к протеолитическим ферментам пищеварительного тракта.
Лекарство проявляет активность по отношению к бычьему цепню (Taeniarhynchus saginatus), широкому лентецу (Diphyllobothrium latum), карликовому цепню (Hymenolepis nana).
Средство предотвращает процессы поглощения паразитами глюкозы, окислительное фосфорилирование и анаэробный метаболизм в целом.
Показания к применению
Лекарство назначают:
- при тениаринхозе, тениозе;
- для лечения дифиллоботриоза и гименолепидоза.
Лечение коронавируса Никлозамидом
В марте, когда началась пандемия коронавируса COVID-19, компания Gero, которая специализируется на разработке лекарственных средств с помощью искусственного интеллекта, применила свою платформу для поиска веществ, потенциально способных бороться с COVID-19. В список таких потенциально полезных для человека при лечении вируса веществ пошли Никлозамид и нитазоксанид (противогельминтное и противоопухолевое средства). Лекарство от гельминтов было одобрено для проведения экспериментального лечения во Франции.
ВОЗ настоятельно рекомендуется не заниматься самолечением и при признаках болезни обращаться к специалистам.
Противопоказания
Применение Никлозамида противопоказано:
- при аллергии на вещество;
- у пациентов с язвенной болезнью желудка, с выраженными нарушениями работы почек и печени;
- при анемии;
- пожилым пациентам;
- беременным женщинам.
Побочные действия
При лечении Никлозамидом могут наблюдаться следующие побочные реакции:
- тошнота и рвота;
- аллергические проявления;
- обострение нейродермита.
Никлозамид, инструкция по применению (Способ и дозировка)
Форма выпуска лекарства — таблетки, принимают внутрь.
Как правило, дозировка для детей и взрослых пациентов от 12 лет составляет по 2-3 грамма в сутки. Для детей от 2 лет — не более 0,5 г в сутки. В возрасте от 5 до 12 лет назначают по 1,5 грамма лекарства в день.
Частота приема зависит от заболевания и типа гельминтоза.
Передозировка
Данные о передозировке средством отсутствуют.
Взаимодействие
Препарат хорошо сочетается с другими лекарственными средствами. Клинически важных взаимодействий не наблюдалось.
Условия продажи
Лекарственное средство может быть не во всех аптеках. Кроме того, чтобы его приобрести могут попросить рецепт на латинском.
Особые указания
Таблетки Никлозамид не рекомендуют применять при свином цепне, при таком лечении повышается риск развития цистицеркоза.
При лечении лекарственным средством рекомендуется принимать легко усваиваемую, жидкую пищу.
Обычно во время лечения дополнительно назначают слабительные препараты.
Аналоги Никлозамида
Совпадения по коду АТХ 4-го уровня:
Торговое название препарата: Yomesan, Фенасал. Других аналогов в России не существует.
Отзывы о Никлозамиде
Отзывов о препарате не много, но из тех что есть, попадаются в основном хорошие. Таблетки Никлозамид чаще всего вызывают побочные реакции, указанные в инструкции: отрыжку, тошноту, повышенное газообразование.
Некоторые пациенты занимались самолечением и возмущаются, почему не помогает такая терапия. Те же, кто пили таблетки строго по схеме и соблюдали все меры предосторожности, успешно избавились от паразитов.
Цена Никлозамида
Купить Никлозамид в Москве в форме таблеток Фенасал можно по цене 2500 рублей за 30 таблеток, дозировкой по 250 мг. Приобрести лекарство в Спб можно чуть дешевле — порядка 2000 рублей за 30 таблеток, 0,25 г. Средство можно купить и в интернет-аптеке при наличии рецепта.
Package insert / product label
Dosage form: tablet, film coated
Drug class: Amebicides
Highlights of Prescribing Information
These highlights do not include all the information needed to use NITAZOXANIDE TABLETS safely and effectively. See full prescribing information for NITAZOXANIDE TABLETS.
NITAZOXANIDE tablets, for oral use
Initial U.S. Approval: 2002
Indications and Usage for Nitazoxanide
Nitazoxanide tablets are antiprotozoal indicated for the treatment of diarrhea caused by Giardia lamblia or Cryptosporidium parvum (1).
Limitations of Use:
Nitazoxanide tablets have not been shown to be effective for the treatment of diarrhea caused by C. parvum in HIV-infected or immunodeficient patients (1).
Nitazoxanide Dosage and Administration
• Nitazoxanide tablets should not be administered to pediatric patient 11 years of age or younger (2.1).
• Dosage for treatment of diarrhea caused by G. lamblia or C. parvum (2.1):
Age |
Dosage |
Duration |
| 12 years and older | One nitazoxanide tablet (500 mg nitazoxanide) every 12 hours with food | 3 days |
Dosage Forms and Strengths
• Nitazoxanide tablets: 500 mg (3.1)
Contraindications
Adverse Reactions/Side Effects
The most common adverse reactions in ≥2% of patients were abdominal pain, headache, chromaturia, and nausea (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharmaceuticals, Inc at 1-866-562-4597 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Competition for binding sites may occur when administered concurrently with other highly plasma protein-bound drugs with narrow therapeutic indices. Monitor for adverse reactions (7).
Use In Specific Populations
Pediatric Patients: Safety and efficacy of ALINIA for Oral Suspension in pediatric patients less than one year of age has not been studied (8.4).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2022
1. Indications and Usage for Nitazoxanide
Diarrhea caused by Giardia lamblia or Cryptosporidium parvum:
Nitazoxanide tablets (patients 12 years and older) are indicated for the treatment of diarrhea caused by Giardia lamblia or Cryptosporidium parvum.
Limitations of Use
Nitazoxanide tablets have not been shown to be effective for the treatment of diarrhea caused by Cryptosporidium parvum in HIV-infected or immunodeficient patients [see Clinical Studies (14.2)].
2. Nitazoxanide Dosage and Administration
2.1 Recommended Dosage and Important Administration Instructions
Important Administration Instructions for Pediatric Patients 11 years of Age or Younger:
Nitazoxanide tablets should not be administered to pediatric patients 11 years of age or younger because a single tablet contains a greater amount of nitazoxanide than the recommended dosing in this pediatric age group.
Table 1. Recommended Dosage
Age |
Dosage |
Duration |
| 12 years and older | One Nitazoxanide tablet (500 mg nitazoxanide) taken orally every 12 hours with food | 3 days |
3. Dosage Forms and Strengths
3.1 Nitazoxanide Tablets (500 mg)
Round, yellow colored film coated tablet, debossed with “SUVEN” on one side and “500” on the other side. Each tablet contains 500 mg of nitazoxanide.
4. Contraindications
4.1 Hypersensitivity
Nitazoxanide tablets are contraindicated in patients with a prior hypersensitivity to nitazoxanide or any other ingredient in the formulations.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of nitazoxanide was evaluated in 2177 HIV-uninfected subjects 12 months of age and older who received nitazoxanide tablets or nitazoxanide for oral suspension at the recommended dose for at least three days. In pooled controlled clinical trials involving 536 HIV-uninfected subjects treated with nitazoxanide tablets or nitazoxanide for oral suspension, the most common adverse reactions were abdominal pain, headache, chromaturia and nausea (≥2%).
Safety data were analyzed separately for 280 HIV-uninfected subjects ≥12 years of age receiving nitazoxanide at the recommended dose for at least three days in 5 placebo-controlled clinical trials and for 256 HIV-uninfected subjects 1 through 11 years of age in 7 controlled clinical trials. There were no differences between the adverse reactions reported for nitazoxanide-treated subjects based upon age.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of nitazoxanide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following is a list of adverse reactions spontaneously reported with nitazoxanide tablets which were not included in clinical trial listings:
Gastrointestinal disorders: diarrhea, gastroesophageal reflux disease
Nervous System disorders: dizziness
Respiratory, thoracic and mediastinal disorders: dyspnea
Skin and subcutaneous tissue disorders: rash, urticaria
7. Drug Interactions
7.1 Highly Protein Bound Drugs with Narrow Therapeutic Indices
Tizoxanide (the active metabolite of nitazoxanide) is highly bound to plasma protein (>99.9%). Therefore, monitor for adverse reactions when administering nitazoxanide concurrently with other highly plasma protein-bound drugs with narrow therapeutic indices, as competition for binding sites may occur (e.g., warfarin).
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no data with nitazoxanide in pregnant women to inform a drug-associated risk. No teratogenicity or fetotoxicity was observed in animal reproduction studies with administration of nitazoxanide to pregnant rats and rabbits during organogenesis at exposures 30 and 2 times, respectively, the exposure at the maximum recommended human dose of 500 mg twice daily based on body surface area (BSA).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Nitazoxanide was administered orally to pregnant rats at doses of 0, 200, 800 or 3200 mg/kg/day on gestation days 6 to 15. Nitazoxanide produced no evidence of systemic maternal toxicity when administered once daily via oral gavage to pregnant female rats at levels up to 3200 mg/kg/day during the period of organogenesis.
In rabbits, nitazoxanide was administered at doses of 0, 25, 50, or 100 mg/kg/day on gestation days 7 to 20. Oral treatment of pregnant rabbits with nitazoxanide during organogenesis resulted in minimal maternal toxicity and no external fetal anomalies.
8.2 Lactation
Risk Summary
No information regarding the presence of nitazoxanide in human milk, the effects on the breastfed infant, or the effects on milk production is available. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for nitazoxanide and any potential adverse effects on the breastfed infant from nitazoxanide or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of ALINIA for Oral Suspension for the treatment of diarrhea caused by G. lamblia or C. parvum in pediatric patients 1 to 11 years of age has been established based on three (3) randomized, controlled studies with 104 pediatric subjects treated with ALINIA for Oral Suspension 100 mg/5 mL. Furthermore, the safety and efficacy of ALINIA for Oral Suspension for the treatment of diarrhea caused by G. lamblia or C. parvum in pediatric patients 12 to 17 years of age has been established based on two (2) randomized controlled studies with 44 pediatric subjects treated with ALINIA for Oral Suspension 100 mg/5 mL. [see Clinical Studies (14.1)]
The safety and efficacy of nitazoxanide tablets for the treatment of diarrhea caused by G. lamblia or C. parvum in pediatric patients 12 to 17 years of age has been established based on three (3) randomized controlled studies with 47 pediatric subjects treated with nitazoxanide tablets 500 mg.
A single nitazoxanide tablet contains a greater amount of nitazoxanide than is recommended for use in pediatric patients 11 years or younger. [see Dosage and Administration (2.1)].
Safety and efficacy of ALINIA for Oral Suspension in pediatric patients less than one year of age has not been studied.
8.5 Geriatric Use
Clinical studies of nitazoxanide tablets and Alinia for oral suspension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing nitazoxanide tablets and Alinia for oral suspension.
8.6 Renal and Hepatic Impairment
The pharmacokinetics of nitazoxanide in patients with compromised renal or hepatic function has not been studied.
8.7 HIV-Infected or Immunodeficient Patients
Nitazoxanide tablets and Alinia for oral suspension have not been studied for the treatment of diarrhea caused by G. lamblia in HIV-infected or immunodeficient patients. Nitazoxanide tablets and Alinia for oral suspension have not been shown to be superior to placebo for the treatment of diarrhea caused by C. parvum in HIV-infected or immunodeficient patients [see Clinical Studies (14)].
10. Overdosage
Limited information on nitazoxanide overdosage is available. In the event of overdose, gastric lavage may be appropriate soon after oral administration. Patients should be observed and given symptomatic and supportive treatment. There is no specific antidote for overdose with nitazoxanide. Because tizoxanide is highly protein bound (>99.9%), dialysis is unlikely to significantly reduce plasma concentrations of the drug.
11. Nitazoxanide Description
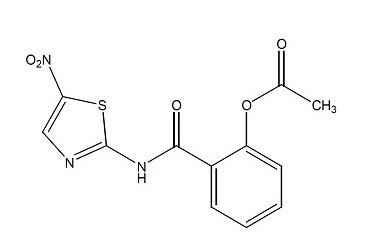
Nitazoxanide tablets contain the active ingredient, nitazoxanide, a synthetic antiprotozoal for oral administration. Nitazoxanide is a pale yellow to yellow crystalline powder. It is poorly soluble in ethanol and practically insoluble in water. Chemically, nitazoxanide is 2-acetyloxy-N-(5-nitro-2-thiazolyl) benzamide. The molecular formula is C12H9N3O5S and the molecular weight is 307.3. The structural formula is:
Nitazoxanide tablets contain 500 mg of nitazoxanide and the following inactive ingredients: maize starch, partially pregelatinized maize starch, sodium starch glycolate, hypromellose, talc, magnesium stearate, soy lecithin, polyvinyl alcohol, xanthan gum, titanium dioxide, D&C Yellow No.10 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake and FD&C Blue No.2 Aluminum Lake.
12. Nitazoxanide — Clinical Pharmacology
12.1 Mechanism of Action
Nitazoxanide is an antiprotozoal [see Microbiology (12.4)].
12.3 Pharmacokinetics
Absorption
Single Dosing:
Following oral administration of nitazoxanide tablets or oral suspension, the parent drug, nitazoxanide, is not detected in plasma. The pharmacokinetic parameters of the metabolites, tizoxanide and tizoxanide glucuronide are shown in Tables 2 and 3 below.
Table 2. Mean (+/- SD) plasma pharmacokinetic parameters of tizoxanide and tizoxanide glucuronide following administration of a single dose of one 500 mg nitazoxanide tablet with food to subjects ≥12 years of age
| Tizoxanide |
Tizoxanide Glucuronide |
|||||
| Age | Cmax (μg/mL) | *Tmax (hr) |
AUCԏ (μg•hr/mL) |
Cmax (μg/mL) |
*Tmax (hr) |
AUCԏ (μg•hr/mL) |
| 12-17 years ≥18 years |
9.1 (6.1) 10.6 (2.0) |
4.0 (1-4) 3.0 (2-4) |
39.5 (24.2) 41.9 (6.0) |
7.3 (1.9) 10.5 (1.4) |
4.0 (2-8) 4.5 (4-6) |
46.5 (18.2) 63.0 (12.3) |
* Tmax is given as a Mean (Range)
Table 3. Mean (+/- SD) plasma pharmacokinetic of tizoxanide and tizoxanide glucuronide parameter values following administration of a single dose of nitazoxanide for oral suspension with food to subjects ≥1 year of age
Tizoxanide |
Tizoxanide Glucuronide |
||||||
| Age | Dose | Cmax (μg/mL) | *Tmax(hr) | AUCinf (μg•hr/mL) | Cmax (μg/mL) | *Tmax(hr) | AUCinf (μg•hr/mL) |
| 1-3 years | 100 mg | 3.11 (2.0) | 3.5 (2-4) | 11.7 (4.46) | 3.64 (1.16) | 4.0 (3-4) | 19.0 (5.03) |
| 4-11 years | 200 mg | 3.00 (0.99) | 2.0 (1-4) | 13.5 (3.3) | 2.84 (0.97) | 4.0 (2-4) | 16.9 (5.00) |
| ≥18 years | 500 mg | 5.49 (2.06) | 2.5 (1-5) | 30.2 (12.3) | 3.21 (1.05) | 4.0 (2.5-6) | 22.8 (6.49) |
* Tmax is given as a Mean (Range)
Multiple dosing:
Following oral administration of a single nitazoxanide tablet every 12 hours for 7 consecutive days, there was no significant accumulation of nitazoxanide metabolites tizoxanide or tizoxanide glucuronide detected in plasma.
Bioavailability:
Nitazoxanide for oral suspension is not bioequivalent to nitazoxanide tablets. The relative bioavailability of the suspension compared to the tablet was 70%.
When nitazoxanide tablets are administered with food, the AUCԏ of tizoxanide and tizoxanide glucuronide in plasma is increased almost two-fold and the Cmax is increased by almost 50%.
When nitazoxanide for oral suspension was administered with food, the AUCԏ of tizoxanide and tizoxanide glucuronide increased by about 45-50% and the Cmax increased by ≤10%.
Nitazoxanide tablets and nitazoxanide for oral suspension were administered with food in clinical trials and hence they are recommended to be administered with food [see Dosage and Administration (2.1)].
Distribution
In plasma, more than 99% of tizoxanide is bound to proteins.
Elimination
Metabolism
Following oral administration in humans, nitazoxanide is rapidly hydrolyzed to an active metabolite, tizoxanide (desacetyl-nitazoxanide). Tizoxanide then undergoes conjugation, primarily by glucuronidation.
Excretion
Tizoxanide is excreted in the urine, bile and feces, and tizoxanide glucuronide is excreted in urine and bile. Approximately two-thirds of the oral dose of nitazoxanide is excreted in the feces and one-third in the urine.
Specific Populations
Pediatric Patients
The pharmacokinetics of tizoxanide and tizoxanide glucuronide following administration of nitazoxanide tablets in pediatric patients 12-17 years of age are provided above in Table 2.Mean (±SD) plasma pharmacokinetic parameters of tizoxanide and tizoxanide glucuronide following administraton of a single dose of one 500 mg Nitazoxanide Tablet with food to subject ≥12 years of age. The pharmacokinetics of tizoxanide and tizoxanide glucuronide following administration of nitazoxanide for oral suspension in pediatric patients 1-11 years of age are provided above in Table 3. Mean (±SD) plasma pharmacokinetic of tizoxanide and tizoxanide glucuronide parameter values following administration of a single dose of ALINIA for Oral suspension with food to subjects ≥1 year of age.
Drug Interaction Studies
In vitro studies demonstrated that tizoxanide has no significant inhibitory effect on cytochrome P450 enzymes.
12.4 Microbiology
Mechanism of Action
The antiprotozoal activity of nitazoxanide is believed to be due to interference with the pyruvate:ferredoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reaction which is essential to anaerobic energy metabolism. Studies have shown that the PFOR enzyme from G. lamblia directly reduces nitazoxanide by transfer of electrons in the absence of ferredoxin. The DNA-derived PFOR protein sequence of C. parvum appears to be similar to that of G. lamblia. Interference with the PFOR enzyme-dependent electron transfer reaction may not be the only pathway by which nitazoxanide exhibits antiprotozoal activity.
Resistance
A potential for development of resistance by C. parvum or G. lamblia to nitazoxanide has not been examined.
Antimicrobial Activity
Nitazoxanide and its metabolite, tizoxanide, are active in vitro in inhibiting the growth of (i) sporozoites and oocysts of C. parvum and (ii) trophozoites of G. lamblia.
Susceptibility Test Methods
For protozoa such as C. parvum and G. lamblia, standardized tests for use in clinical microbiology laboratories are not available.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis
Long-term carcinogenicity studies have not been conducted.
Mutagenesis
Nitazoxanide was not genotoxic in the Chinese hamster ovary (CHO) cell chromosomal aberration assay or the mouse micronucleus assay. Nitazoxanide was genotoxic in one tester strain (TA 100) in the Ames bacterial mutation assay.
Impairment of Fertility
Nitazoxanide did not adversely affect male or female fertility in the rat at 2400 mg/kg/day (approximately 20 times the clinical adult dose adjusted for body surface area).
14. Clinical Studies
14.1 Diarrhea Caused by G. lamblia
Diarrhea caused by G. lamblia in adults and adolescents 12 years of age or older:
In a double-blind, controlled trial (Study 1) conducted in Peru and Egypt in adults and adolescents with diarrhea and with one or more enteric symptoms (e.g., abdominal pain, nausea, vomiting, fever, abdominal distention, loss of appetite, flatulence) caused by G. lamblia, a three-day course of treatment with nitazoxanide tablets administered 500 mg twice daily was compared with a placebo tablet for 3 days. A third group of patients received open-label nitazoxanide for oral suspension administered 500 mg/25 mL of suspension twice daily for 3 days. A second double-blind, controlled trial (Study 2) conducted in Egypt in adults and adolescents with diarrhea and with or without enteric symptoms (e.g., abdominal colic, abdominal tenderness, abdominal cramps, abdominal distention, fever, bloody stool) caused by G. lamblia compared nitazoxanide tablets administered 500 mg twice daily for 3 days to a placebo tablet. For both of these studies, clinical response was evaluated 4 to 7 days following the end of treatment. A clinical response of ‘well’ was defined as ‘no symptoms, no watery stools and no more than 2 soft stools with no hematochezia within the past 24 hours’ or ‘no symptoms and no unformed stools within the past 48 hours.’ The following clinical response rates were obtained:
Table 4. Adult and Adolescent Patients with Diarrhea Caused by G. lamblia
Clinical Response Rates* 4 to 7 Days Post-therapy
% (Number of Successes/Total)
| Nitazoxanide Tablets |
Nitazoxanide for Oral Suspension |
Placebo Tablets |
|
| Study 1 | 85% (46/54) ¶ § |
83% (45/54) ¶ § |
44% (12/27) |
| Study 2 | 100% (8/8) | — | 30% (3/10) |
*Includes all patients randomized with G. lamblia as the sole pathogen. Patients failing to complete the studies were treated as failures.
¶Clinical response rates statistically significantly higher when compared to placebo.
§The 95% confidence interval of the difference in response rates for the tablet and suspension is (-14%, 17%).
Some patients with ‘well’ clinical responses had G. lamblia cysts in their stool samples 4 to 7 days following the end of treatment. The relevance of stool examination results in these patients is unknown. Patients should be managed based upon clinical response to treatment.
Diarrhea caused by G. lamblia in pediatric patients 1 through 11 years of age:
In a randomized, controlled trial conducted in Peru in 110 pediatric patients with diarrhea and with or without enteric symptoms (e.g., abdominal distention, right iliac fossa tenderness) caused by G. lamblia, a three-day course of treatment with nitazoxanide (100 mg twice daily in pediatric patients ages 24-47 months, 200 mg twice daily in pediatric patients ages 4 through 11 years) was compared to a five-day course of treatment with metronidazole (125 mg twice daily in pediatric patients ages 2 through 5 years, 250 mg twice daily in pediatric patients ages 6 through 11 years). Clinical response was evaluated 7 to 10 days following initiation of treatment with a ‘well’ response defined as ‘no symptoms, no watery stools and no more than 2 soft stools with no hematochezia within the past 24 hours’ or ‘no symptoms and no unformed stools within the past 48 hours.’ The following clinical response rates were obtained:
Table 5. Clinical Response Rates in Pediatric Patients 7 to 10 Days Following Initiation of Therapy
Intent-to-Treat and Per Protocol Analyses
% (Number of Successes/Total), [95% Confidence Interval]
| Population |
Nitazoxanide (3 days) |
Metronidazole (5 days) |
95% CI Diff§ |
| Intent-to-treat analysis† |
85% (47/55) | 80% (44/55) | [-9%, 20%] |
| Per protocol analysis¶ |
90% (43/48) | 83% (39/47) | [-8%, 21%] |
†Intent-to-treat analysis includes all patients randomized with patients not completing the study treated as failures.
¶Per protocol analysis includes only patients who took all of their medication and completed the study. Seven patients in each treatment group missed at least one dose of medication and one in the metronidazole treatment group was lost to follow-up.
§95% Confidence Interval on the difference in response rates (nitazoxanide-metronidazole).
Some patients with ‘well’ clinical responses had G. lamblia cysts in their stool samples 4 to 7 days following the end of treatment. The relevance of stool examination results in these patients is unknown. Patients should be managed based upon clinical response to treatment.
14.2 Diarrhea Caused by C. parvum
Diarrhea caused by C. parvum in adults and adolescents 12 years of age or older:
In a double-blind, controlled trial conducted in Egypt in adults and adolescents with diarrhea and with or without enteric symptoms (e.g., abdominal pain/cramps, nausea, vomiting) caused by C. parvum, a three-day course of treatment with nitazoxanide tablets administered 500 mg twice daily was compared with a placebo tablet for 3 days. A third group of patients received open-label nitazoxanide for oral suspension administered 500 mg/25 mL of suspension twice daily for 3 days. Clinical response was evaluated 4 to 7 days following the end of treatment. A clinical response of ‘well’ was defined as ‘no symptoms, no watery stools and no more than 2 soft stools within the past 24 hours’ or ‘no symptoms and no unformed stools within the past 48 hours.’ The following clinical response rates were obtained:
Table 6. Clinical Response Rates in Adult and Adolescent Patients 4 to 7 Days Post-therapy
% (Number of Successes/Total)
| Nitazoxanide Tablets |
Nitazoxanide Suspension |
Placebo Tablets |
|
| Intent-to-treat analysis* | 96% (27/28) ¶ § |
87% (27/31) ¶ § |
41% (11/27) |
*Includes all patients randomized with C. parvum as the sole pathogen. Patients failing to complete the study were treated as failures.
¶Clinical response rates statistically significantly higher when compared to placebo.
§The 95% confidence interval of the difference in response rates for the tablet and suspension is ( -10%, 28%).
In a second double-blind, placebo-controlled trial of nitazoxanide tablets conducted in Egypt in adults and adolescents with diarrhea and with or without enteric symptoms (e.g., abdominal colic, abdominal cramps, epigastric pain) caused by C. parvum as the sole pathogen, clinical and parasitological response rates showed a similar trend to the first study. Clinical response rates, evaluated 2 to 6 days following the end of treatment, were 71% (15/21) in the nitazoxanide group and 42.9% (9/21) in the placebo group.
Some patients with ‘well’ clinical responses had C. parvum oocysts in their stool samples 4 to 7 days following the end of treatment. The relevance of stool examination results in these patients is unknown. Patients should be managed based upon clinical response to treatment.
Diarrhea caused by C. parvum in pediatric patients 1 through 11 years of age:
In two double-blind, controlled trials in pediatric patients with diarrhea and with or without enteric symptoms (e.g., abdominal distention, colic, left iliac fossa tenderness) caused by C. parvum, a three-day course of treatment with nitazoxanide (100 mg twice daily in pediatric patients ages 12-47 months, 200 mg twice daily in pediatric patients ages 4 through 11 years) was compared with a placebo. One study was conducted in Egypt in outpatients ages 1 through 11 years with diarrhea caused by C. parvum. Another study was conducted in Zambia in malnourished pediatric patients admitted to the hospital with diarrhea caused by C. parvum. Clinical response was evaluated 3 to 7 days post-therapy with a ‘well’ response defined as ‘no symptoms, no watery stools and no more than 2 soft stools within the past 24 hours’ or ‘no symptoms and no unformed stools within the past 48 hours.’ The following clinical response rates were obtained:
Table 7. Clinical Response Rates in Pediatric Patients 3 to 7 Days Post-therapy Intent-to-Treat Analyses
% (Number of Successes/Total)
| Population | Nitazoxanide* | Placebo |
| Outpatient Study, age 1 -11 years | 88% (21/24) | 38% (9/24) |
| Inpatient Study, Malnourished¶, age 12-35 months | 56% (14/25) | 23% (5/22) |
*Clinical response rates statistically significantly higher compared to placebo.
¶60% considered severely underweight, 19% moderately underweight, 17% mild underweight.
Some patients with ‘well’ clinical responses had C. parvum oocysts in their stool samples 3 to 7 days following the end of treatment. The relevance of stool examination results in these patients is unknown. Patients should be managed based upon clinical response to treatment.
Diarrhea caused by C. parvum in Acquired Immune Deficiency Syndrome (AIDS) patients:
A double-blind, placebo-controlled trial did not produce clinical cure rates that were significantly different from the placebo control when conducted in hospitalized, severely malnourished pediatric patients with acquired immune deficiency syndrome (AIDS) in Zambia. In this study, the pediatric patients received a three day course of nitazoxanide suspension (100 mg twice daily in pediatric patients ages 12-47 months, 200 mg twice daily in pediatric patients ages 4 through 11 years) and were evaluated for response four days after the end of treatment.
16. How is Nitazoxanide supplied
16.1 Nitazoxanide Tablets (500 mg)
Nitazoxanide tablets are round, yellow colored film coated tablet, debossed with “SUVEN” on one side and “500” on the other side. Each tablet contains 500 mg of nitazoxanide. The tablets are packaged in HDPE bottles of 6, 12, 18 and 30 tablets.
| Bottles of 6 tablets | NDC 64980-526-60 |
| Bottles of 12 tablets | NDC 64980-526-21 |
| Bottles of 18 tablets | NDC 64980-526-81 |
| Bottles of 30 tablets | NDC 64980-526-03 |
Store the tablets at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F). [See USP Controlled Room Temperature]
17. Patient Counseling Information
Advise patients and parents/caregivers of pediatric patients taking nitazoxanide tablets of the following information:
Dosage and Administration:
Nitazoxanide tablets should be taken with food.
Drug-drug Interactions:
Avoid concurrent warfarin use.
Manufactured by:
Suven Pharmaceuticals Limited
Pashamylaram, Telangana 502307, India
Manufactured for:
Rising Pharmaceuticals, Inc.
East Brunswick, NJ 08816
ALINIA for Oral Suspension is distributed by Lupin Pharmaceuticals, Inc. under license from Romark.
ALINIA is a registered trademark of Romark
ML No. 24/MD/AP/2009/F/CC
Revised: 02/2022
PIR52603-03
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Nitazoxanide
Tablets
500 mg
6 Tablets Rx only

Rising® NDC 64980-526-21
Nitazoxanide
Tablets
500 mg
12 Tablets Rx only

Rising® NDC 64980-526-81
Nitazoxanide
Tablets
500 mg
18 Tablets Rx only

Rising® NDC 64980-526-03
Nitazoxanide
Tablets
500 mg
30 Tablets Rx only

Торговое название:
Наназоксид
Nanazoxid
Состав:
Каждая таблетка содержит:
Нитазоксанид 500 мг
Вспомогательные компоненты:
Повидон, авицел, кроскармеллоза натрия, тальк, магния стеарат, гидроксипропил метилцеллюлоза, титана диоксид.
Свойства:
Синтетическое лекарство, производное сиаликамида, употребляющееся как противопаразитарное средство широкого спектра действия, эффективность которого подтверждена в отношении простейших и гельминтов. Рекомендовано к использованию при инфицировании Cryptosporidium parvum и Giardia lamblia пациентов в возрасте до 1 года.
Противогельминтное действие препарата проявляется через поглощение фермента, необходимого для жизнедеятельности паразитов, в частности, препятствует полимеризации белка тубулина у гельминтов. В отношении простейших его действие проявляется другим образом: биохимические исследования и исследования с применением излучения электронов продемонстрировали, что 5-нитрогруппа метронидазола и в меньшей степени гидрогеназа уменьшает количество ферредоксина. В отношении простейших Наназоксид действует как токсический радикал в метаболизме углеводов. Также отмечена его эффективность в отношении вируса гриппа А, в этом случае он препятствует внедрению чужеродного белка в мембрану клетки хозяина.
Показания:
Таблетки и суспензию применяют для терапии паразитарных инфекций, где возбудителями выступают простейшие (лямблиоз, амёбная, инфузорная дизентерия, диентамобиаз, урогенитальный трихомониаз), кокцидии (криптоспородиоз, изоспороз, циклоспороз) и вирусы. Эффективен при гельминтозах. Особенно чувствительны к нему аскариды, трематода фасциола обыкновенная, фасциола гигантика, бычий цепень.
Сироп принимают дети 1 года и старше.
Таблетки применяют только для пациентов, достигших 12-ти летнего возраста.
Способ применения и дозы:
Дети 1-3 лет принимают по 5 мл сиропа каждые 12 часов с едой.
Дети 4-11 лет принимают по 10 мл сиропа каждые 12 часов с едой.
Дети старше 12 лет и взрослые принимают по 1 таблетке или 25 мл сиропа каждые 12 часов с едой.
Курс лечения длится 3 дня.
Противопоказания:
Повышения чувствительность к нитазоксаниду и другим компонентам препарата.
Меры предосторожности:
Перед началом приема данного препарата сообщите врачу об уже используемых медикаментах, пищевых добавках (например, витаминах, натуральным добавкам и др. ), аллергических реакциях, существующих заболеваниях и текущем состоянии здоровья (например, беременность, предстоящая операция и др. ).
Побочные эффекты:
Наиболее часто нарушения пищеварительной системы, боли в животе, рвота и диарея. Могут возникать головные боли.
Способ хранения:
В сухом прохладном месте при температуре не выше 30 градусов.
Упаковка:
Картонная коробка вмещает 3 блистера по 6 таблеток, бумажную инструкцию.

Нитазоксанид
Кат. №: N052-50MG
Цена По запросу
Количество
Вы уже добавили максимально доступное на складе кол-во товара
Достигнуто максимально доступное кол-во
Под заказ
{{!!storageProduct ? ‘На складе’ : ‘Под заказ’}}
Ожидается поставка
Nitazoxanide is a thiazolid drug used primarily in the treatment of Giardia intestinalis infection in humans. In addition to its antiparasitic activity, it as also demonstrated promise against a broad spectrum of gram-postive and gram-negative bacterial pathogens, including both aerobic and anaerobic species.Nitazoxanide is soluble in DMSO, poorly soluble in ethanol, and practically insoluble in water.
-
Молекулярный вес
307,28 -
Скачать каталог «ХИММЕД» в формате pdf

Химические реактивы

Лабораторное оборудование

Аналитическое оборудование

Биохимия

Проектирование лабораторий

Материалы для микроэлектроники
Для уточнения данных о стоимости и наличии товаров, пожалуйста, обращайтесь к
менеджерам по продажам.
Похоже, что-то пошло не так.
Попробуйте перезагрузить страницу.
×
Авторизация прошла успешно.
