
CX50 U l t r a s o u n d S y s t e m
User Manual
4535 616 38521 Rev B
5HYLVLRQ
© 2012 Koninklijke Philips Electronics N.V. All rights reserved. Published in USA.
Manufactured by Philips Ultrasound
22100 Bothell-Everett Highway
Bothell, WA 98021-8431
USA
Telephone: +1 425-487-7000 or 800-426-2670
Fax: +1 425-485-6080
www.healthcare.philips.com/ultrasound
This Medical Device meets the provisions of the transposition of the Medical
Device Directive 93/42/EEC within the country of origin of the Notified Body
concerned with the device.
European Union Representative
Philips Medical Systems Nederland B.V.
Quality & Regulatory Affairs
Veenpluis 4-6
5684PC Best
The Netherlands
WARNING
United States federal law restricts this device to sale by or on the order of a
physician.
This document and the information contained in it is proprietary and confidential information of Philips Healthcare
(«Philips») and may not be reproduced, copied in whole or in part, adapted, modified, disclosed to others, or disseminated
without the prior written permission of the Philips Legal Department. This document is intended to be used by customers
and is licensed to them as part of their Philips equipment purchase. Use of this document by unauthorized persons is
strictly prohibited.
Philips provides this document without warranty of any kind, implied or expressed, including, but not limited to, the
implied warranties of merchantability and fitness for a particular purpose.
Philips has taken care to ensure the accuracy of this document. However, Philips assumes no liability for errors or
omissions and reserves the right to make changes without further notice to any products herein to improve reliability,
function, or design. Philips may make improvements or changes in the products or programs described in this document
at any time.
Unauthorized copying of this document, in addition to infringing copyright, might reduce the ability of Philips to provide
accurate and current information to users.
This product may contain remanufactured parts equivalent to new in performance, or parts that have had incidental use.
Philips Ultrasound products may be manufactured under or operate in accordance with one or more of the following
United States patents and corresponding patents in other countries: U.S. Patent Numbers 5,798,461; 6,450,958; 6,471,649;
6,527,721; 6,540,685; 6,572,547; 6,679,849. Other patent applications are pending in various countries.
«Chroma,» «Color Power Angio,» «High Q,» «QLAB,» «SonoCT,» and «XRES» are trademarks of Koninklijke Philips
Electronics N.V.
Non-Philips product names may be trademarks of their respective owners.
CX50 User Manual
2
4535 616 38521

Contents
1 Read This First………………………………………………………………………………15
Intended Audience………………………………………………………………………………………………15
Intended Use………………………………………………………………………………………………………..15
Warnings………………………………………………………………………………………………………………16
Warning Symbols…………………………………………………………………………………………………17
User Information Components…………………………………………………………………………..17
Product Conventions…………………………………………………………………………………………..18
User Information Conventions……………………………………………………………………………19
Upgrades and Updates………………………………………………………………………………………..21
Customer Comments………………………………………………………………………………………….21
Supplies and Accessories…………………………………………………………………………………….21
Customer Service………………………………………………………………………………………………..22
Recycling, Reuse, and Disposal……………………………………………………………………………22
2 Safety……………………………………………………………………………………………25
Basic Safety…………………………………………………………………………………………………………..25
Electrical Safety……………………………………………………………………………………………………26
Defibrillators…………………………………………………………………………………………………..29
Fire Safety……………………………………………………………………………………………………….30
Mechanical Safety…………………………………………………………………………………………………30
Equipment Protection………………………………………………………………………………………….31
Product Compatibility………………………………………………………………………………………….32
Symbols………………………………………………………………………………………………………………..33
Biological Safety……………………………………………………………………………………………………42
FDA Medical Alert on Latex…………………………………………………………………………..44
ALARA Education Program……………………………………………………………………………46
Output Display……………………………………………………………………………………………….50
Control Effects………………………………………………………………………………………………..54
3
CX50 User Manual
4535 616 38521

Related Guidance Documents…………………………………………………………………………..57
Acoustic Output and Measurement………………………………………………………………….57
Acoustic Output Tables…………………………………………………………………………………….61
Acoustic Measurement Precision and Uncertainty…………………………………………..61
Operator Safety………………………………………………………………………………………………………63
Repetitive Strain Injury …………………………………………………………………………………….63
Philips Transducers…………………………………………………………………………………………….63
Glutaraldehyde Exposure…………………………………………………………………………………..64
Infection Control……………………………………………………………………………………………….64
Electromagnetic Compatibility ………………………………………………………………………………66
Radio-Frequency Emissions……………………………………………………………………………….67
ECG Signal………………………………………………………………………………………………………….68
Electrostatic Discharge Precautions………………………………………………………………….69
Electromagnetic Emissions………………………………………………………………………………..70
Approved Cables for Electromagnetic Compliance………………………………………….70
Approved Transducers for Electromagnetic Compliance…………………………………71
Approved Accessories for Electromagnetic Compliance…………………………………72
Electromagnetic Immunity…………………………………………………………………………………72
Electromagnetic Interference…………………………………………………………………………….75
Recommended Separation Distance…………………………………………………………………78
Avoiding Electromagnetic Interference……………………………………………………………..80
Use Restrictions Due to Interference………………………………………………………………81
3 System Overview…………………………………………………………………………….83
System Capabilities…………………………………………………………………………………………………83
Measurements……………………………………………………………………………………………………83
Transducer Types……………………………………………………………………………………………….84
Image Acquisition and Review…………………………………………………………………………..84
Patient Data Protection…………………………………………………………………………………….85
System Options………………………………………………………………………………………………………85
Imaging Options…………………………………………………………………………………………………85
CX50 User Manual
4
4535 616 38521
Contents

Connectivity Options………………………………………………………………………………………..86
Clinical/Analysis Options…………………………………………………………………………………..86
Calculations………………………………………………………………………………………………………..87
QLAB Advanced Quantification Software Options………………………………………….87
Stress Echocardiography……………………………………………………………………………………88
Data Security……………………………………………………………………………………………………..88
System Components………………………………………………………………………………………………88
Video Monitor……………………………………………………………………………………………………90
Control Panel…………………………………………………………………………………………………….90
On/Off (Power) Control……………………………………………………………………………………91
Data Storage ……………………………………………………………………………………………………..92
Peripherals………………………………………………………………………………………………………….93
Transducer Receptacles and Cable Management……………………………………………..93
Physio (ECG) Receptacles…………………………………………………………………………………95
USB Hub…………………………………………………………………………………………………………….96
Wheel Controls…………………………………………………………………………………………………97
4 Preparing the System………………………………………………………………………99
Connecting Devices………………………………………………………………………………………………..99
External Printers………………………………………………………………………………………………100
Connecting an External Printer………………………………………………………………………102
Configuring Local Printers………………………………………………………………………………103
Connecting the Optional Foot Switch……………………………………………………………104
Configuring the Foot Switch……………………………………………………………………………104
Connecting an External Color Monitor ………………………………………………………..104
Attaching the System……………………………………………………………………………………………105
Removing the System……………………………………………………………………………………………106
System Configuration……………………………………………………………………………………………106
Standard Network Support…………………………………………………………………………….107
DICOM Networking Option…………………………………………………………………………..107
Configuration Information………………………………………………………………………………107
5
CX50 User Manual
4535 616 38521
Contents

Entering System Network Settings…………………………………………………………………109
Changing the PC Name…………………………………………………………………………………..111
Wireless Networking………………………………………………………………………………………112
Configuring Wireless Network Properties…………………………………………………….112
Enabling a Wireless Network Connection……………………………………………………..115
Removing a Wireless Network……………………………………………………………………….118
Troubleshooting Wireless Network Connections…………………………………………119
Remote Access………………………………………………………………………………………………..119
Enabling a Remote Access Session………………………………………………………………….120
Repairing Network Connections…………………………………………………………………….120
Moving the System………………………………………………………………………………………………..121
Preparing and Moving………………………………………………………………………………………122
Setting Up After Moving………………………………………………………………………………….123
5 Using the System…………………………………………………………………………..125
Turning the System On and Off……………………………………………………………………………125
Setting the System Time and Date……………………………………………………………………….126
System Cart…………………………………………………………………………………………………………..127
Installing the AC Adapter………………………………………………………………………………..127
Attaching the System……………………………………………………………………………………….130
Removing the System………………………………………………………………………………………130
Adjusting Cart Height……………………………………………………………………………………..130
Using the Wheel Controls………………………………………………………………………………132
Monitor Settings……………………………………………………………………………………………………133
Changing the Monitor Tint……………………………………………………………………………..134
Changing the Monitor Brightness……………………………………………………………………134
System Controls……………………………………………………………………………………………………135
Control Panel…………………………………………………………………………………………………..135
Control Status………………………………………………………………………………………………….136
Changing Control Panel Brightness…………………………………………………………………137
Enabling Automatic Brightness Control …………………………………………………………138
CX50 User Manual
6
4535 616 38521
Contents

Quick Key Controls…………………………………………………………………………………………138
Using Quick Key Controls………………………………………………………………………………139
System Keyboard……………………………………………………………………………………………..139
Typing Special Characters………………………………………………………………………………..140
Typing Accented Characters……………………………………………………………………………140
Status Icons………………………………………………………………………………………………………141
Power Management………………………………………………………………………………………………143
Battery and AC Indicators………………………………………………………………………………144
Changing Power Management Settings……………………………………………………………145
AC Adapter Operation ……………………………………………………………………………………….146
AC Adapter Indicator………………………………………………………………………………………147
Using the AC Adapter……………………………………………………………………………………..147
Battery Operation………………………………………………………………………………………………..148
Installing the Battery ………………………………………………………………………………………149
System Security…………………………………………………………………………………………………….151
Logging On to the System……………………………………………………………………………….151
Logging Off of the System……………………………………………………………………………….151
System and Data Security………………………………………………………………………………..152
Emergency Studies………………………………………………………………………………………………..153
Temporary ID…………………………………………………………………………………………………..153
Starting Emergency Studies……………………………………………………………………………..154
Imaging Display……………………………………………………………………………………………………..155
Image Size Settings………………………………………………………………………………………………..157
Transducer Receptacles and Cable Management…………………………………………………157
Connecting Transducers………………………………………………………………………………….159
Selecting a Transducer……………………………………………………………………………………..161
Selecting a Preset…………………………………………………………………………………………….162
Using Presets……………………………………………………………………………………………………162
Physio Feature……………………………………………………………………………………………………….163
DVD, CD, and USB Devices…………………………………………………………………………………164
7
CX50 User Manual
4535 616 38521
Contents

Media Compatibility…………………………………………………………………………………………164
Loading and Ejecting a Disc……………………………………………………………………………..165
USB Devices…………………………………………………………………………………………………….165
Erasing a DVD or USB Device………………………………………………………………………..167
6 Customizing the System………………………………………………………………..169
Presets…………………………………………………………………………………………………………………..169
Clinical Options and Predefined Presets…………………………………………………………169
Custom Presets……………………………………………………………………………………………….170
Creating Custom Presets………………………………………………………………………………..170
Modifying Custom Presets………………………………………………………………………………171
Deleting Custom Presets………………………………………………………………………………..172
Setting Up Autoselect Presets…………………………………………………………………………172
Presets Menu……………………………………………………………………………………………………173
Using the Presets Menu…………………………………………………………………………………..173
Modifying the Presets Menu…………………………………………………………………………….174
Copying Custom Presets…………………………………………………………………………………175
System Setups……………………………………………………………………………………………………….175
Changing Setups……………………………………………………………………………………………….176
Options…………………………………………………………………………………………………………………176
Installing Temporary Options………………………………………………………………………….176
7 Performing a Study………………………………………………………………………..179
New Patient Studies……………………………………………………………………………………………..179
Entering Patient Data Manually (Without Worklist)………………………………………180
Using Modality Worklist………………………………………………………………………………….181
Selecting a Transducer………………………………………………………………………………………….182
Imaging Modes………………………………………………………………………………………………………182
Using 2D Mode………………………………………………………………………………………………..183
Annotation…………………………………………………………………………………………………………….184
Placing a System-Defined Label on the Display………………………………………………184
Typing a Label on the Display………………………………………………………………………….185
CX50 User Manual
8
4535 616 38521
Contents

Placing a Body Marker on the Display…………………………………………………………….185
Printing………………………………………………………………………………………………………………….186
Review…………………………………………………………………………………………………………………..186
Starting Review………………………………………………………………………………………………..187
Navigating Thumbnails and Images………………………………………………………………….187
Acquiring Images and Loops ……………………………………………………………………………….188
Measurement and Analysis……………………………………………………………………………………189
Performing a 2D Distance Measurement………………………………………………………..190
Obtaining a Typical Labeled Measurement……………………………………………………..190
Obtaining a Calculation Result………………………………………………………………………..191
Ending a Study……………………………………………………………………………………………………….191
8 Transducers……………………………………………………………………………………193
Selecting a Transducer………………………………………………………………………………………….194
Selecting a Preset………………………………………………………………………………………………….194
Clinical Options and Transducers………………………………………………………………………..195
Indications for Use and Supporting Transducers…………………………………………………196
Transducer Maintenance……………………………………………………………………………………….198
Acoustic Artifacts…………………………………………………………………………………………………199
Acoustic Artifacts in 3D Imaging…………………………………………………………………….202
Transducer Covers……………………………………………………………………………………………….204
Transducer Storage……………………………………………………………………………………………….205
Storage for Transport …………………………………………………………………………………….205
Daily and Long-Term Storage………………………………………………………………………….205
9 Endocavity Transducers………………………………………………………………….207
Operators of Endocavity Transducers…………………………………………………………………207
Patient Safety During Endocavity Studies…………………………………………………………….207
Preparing Transducers for Endocavity Use………………………………………………………….208
C9-3v Description………………………………………………………………………………………………..209
C10-3v Description………………………………………………………………………………………………210
Patient-Contact Parts……………………………………………………………………………………………211
9
CX50 User Manual
4535 616 38521
Contents

Biopsy with Endocavity Transducers…………………………………………………………………….212
10 Transesophageal Transducers…………………………………………………………213
Operators of TEE Transducers…………………………………………………………………………….213
Patient Safety During TEE Studies………………………………………………………………………..213
Patient-Contact Parts………………………………………………………………………………………218
Preventing TEE Transducer Problems………………………………………………………………….219
Electrical Safety and TEE Transducers………………………………………………………………….221
Leakage Current and TEE Transducers…………………………………………………………..221
Reducing Risks of Using TEE Transducers………………………………………………………221
TEE Deflection Control Basics ……………………………………………………………………………222
Connecting a TEE Transducer………………………………………………………………………………224
X7-2t TEE Transducer Description……………………………………………………………………..224
TEE Transducer Components………………………………………………………………………………225
TEE Deflection Controls…………………………………………………………………………………227
Manipulating the TEE Tip…………………………………………………………………………………229
Rotating the TEE Image Plane ………………………………………………………………………..231
Checking the TEE Transducer…………………………………………………………………………232
Special Considerations for TEE Studies……………………………………………………………….233
Patient Selection for TEE Transducer Use……………………………………………………..233
Preparing Patients for TEE Studies………………………………………………………………….234
TEE Study Guidelines………………………………………………………………………………………235
Tip Fold-Over……………………………………………………………………………………………………….236
Recognizing Tip Fold-Over……………………………………………………………………………..236
Correcting Tip Fold-Over……………………………………………………………………………….236
Preventing Tip Fold-Over ……………………………………………………………………………….236
TEE Temperature Sensing…………………………………………………………………………………….237
Ensuring Safe TEE Temperatures…………………………………………………………………….238
Manual Auto-Cool Feature……………………………………………………………………………..239
Patient Temperature………………………………………………………………………………………..240
Entering Patient Temperature………………………………………………………………………….240
CX50 User Manual
10
4535 616 38521
Contents

Temperature Display………………………………………………………………………………………..241
Customizing the Temperature Display……………………………………………………………241
Resuming Imaging After Auto-Cool………………………………………………………………..242
Patient Care After a TEE Study……………………………………………………………………………243
TEE Accessories and Supplies………………………………………………………………………………243
Bite Guards………………………………………………………………………………………………………243
TEE Transducer Covers…………………………………………………………………………………..244
Tip Protectors………………………………………………………………………………………………….244
Disposable Drapes…………………………………………………………………………………………..244
TEE Leakage Current Test……………………………………………………………………………………244
TEE Test Background……………………………………………………………………………………….245
Testing TEE Transducer Leakage Current……………………………………………………….247
TEE Transducer References………………………………………………………………………………….248
11 Intraoperative Transducers……………………………………………………………249
Operators of Intraoperative Transducers……………………………………………………………249
Intended Uses for Intraoperative Transducers…………………………………………………….250
Patient Safety During Intraoperative Studies ………………………………………………………250
Patient-Contact Parts………………………………………………………………………………………251
Preventing Intraoperative Transducer Problems ………………………………………………..251
C9-3io Description……………………………………………………………………………………………….252
L10-4lap Description…………………………………………………………………………………………….254
L15-7io Description……………………………………………………………………………………………..256
Preparing Transducers for Intraoperative Use…………………………………………………….257
Disposable Drapes…………………………………………………………………………………………..258
Accessories for Intraoperative Transducers…………………………………………………..258
Electrical Safety and Intraoperative Transducers…………………………………………………258
Leakage Current Testing for Intraoperative Transducers……………………………………259
Testing Intraoperative Transducer Leakage Current (Source)……………………….264
Testing Intraoperative Transducer Leakage Current (Sink)……………………………264
12 ICE Catheter Transducer……………………………………………………………….267
11
CX50 User Manual
4535 616 38521
Contents

Connecting the ICE Catheter………………………………………………………………………………267
13 Biopsy Guides………………………………………………………………………………..269
Attaching and Removing a Biopsy Guide…………………………………………………………….269
Biopsy Guideline Display………………………………………………………………………………………270
Displaying the Biopsy Guideline………………………………………………………………………271
Moving the Needle Length Crosshair……………………………………………………………..272
Biopsy Guideline Quick Keys………………………………………………………………………….272
Biopsy Guide Alignment……………………………………………………………………………………….273
Preparation for Alignment Verification……………………………………………………………273
Verifying the Biopsy Guide Alignment…………………………………………………………….274
Performing a Biopsy Procedure……………………………………………………………………………276
Biopsy Guide Maintenance……………………………………………………………………………………277
Needle Visualization……………………………………………………………………………………………..278
Using Needle Visualization………………………………………………………………………………278
14 Transducer Care……………………………………………………………………………281
Transducer Care Safety………………………………………………………………………………………..281
Latex Product Alert…………………………………………………………………………………………282
Transmissible Spongiform Encephalopathy……………………………………………………..282
Acoustic Coupling Medium………………………………………………………………………………….283
Choosing a Disinfectant………………………………………………………………………………………..283
General Cleaning for All Transducers………………………………………………………………….284
Cleaning a Transducer……………………………………………………………………………………..285
Disinfecting Transducers Using a Wipe or Spray Method ………………………………….285
Cleaning and Disinfecting Cables and Connectors……………………………………………..288
Disinfection of Transducers by Immersion (High-Level Disinfection)………………..291
Disinfecting Transducers by Immersion………………………………………………………….292
Disinfecting TEE Transducers by Immersion…………………………………………………..294
Disinfecting TEE Transducers in an Automated Disinfector………………………….297
Sterilizing Transducers………………………………………………………………………………………….300
Disinfectants Compatibility…………………………………………………………………………………..302
CX50 User Manual
12
4535 616 38521
Contents

Disinfectant Types……………………………………………………………………………………………303
Factors Affecting Disinfectant Efficiency…………………………………………………………303
Disinfectants and Cleaning Solutions Compatibility Table……………………………..304
Gels Compatibility………………………………………………………………………………………………..311
15 System Maintenance……………………………………………………………………..313
Cleaning and Maintaining the System…………………………………………………………………..313
Cleaning the System and ECG Equipment……………………………………………………..313
Disinfectants for System Surfaces……………………………………………………………………315
Disinfecting System Surfaces……………………………………………………………………………315
Cleaning the Trackball……………………………………………………………………………………..316
Cleaning the Battery………………………………………………………………………………………..317
Cleaning the Adapter………………………………………………………………………………………317
Transducer Maintenance……………………………………………………………………………………….318
Printer Maintenance……………………………………………………………………………………………..319
Troubleshooting…………………………………………………………………………………………………….319
Error Messages……………………………………………………………………………………………………..320
For Assistance……………………………………………………………………………………………………….321
16 Specifications…………………………………………………………………………………323
Safety and Regulatory Requirements…………………………………………………………………..327
Index……………………………………………………………………………………………..329
13
CX50 User Manual
4535 616 38521
Contents

CX50 User Manual
14
4535 616 38521
Contents

1 Read This First
This manual is intended to assist you with the safe and effective operation of
your Philips product. Before attempting to operate the product, read this
manual and strictly observe all warnings and cautions. Pay special attention to
the information in the
«Safety» section.
The user information for your Philips product describes the most extensive
configuration of the product, with the maximum number of options and
accessories. Some functions described may be unavailable on your product’s
configuration.
Intended Audience
Before you use your user information, you need to be familiar with ultrasound
techniques. Sonography training and clinical procedures are not included here.
This document is intended for sonographers, physicians, and biomedical
engineers who operate and maintain your Philips product.
Intended Use
This product is intended to be installed, used, and operated only in accordance
with the safety procedures and operating instructions given in the product
user information, and only for the purposes for which it was designed. For
indications for use, see
«Indications for Use and Supporting Transducers» on
page 196
. However, nothing stated in the user information reduces your
responsibility for sound clinical judgment and best clinical procedure.
Installation, use, and operation of this product is subject to the law in the
jurisdictions in which the product is used. Install, use, and operate the product
only in such ways that do not conflict with applicable laws or regulations, which
have the force of law.
15
CX50 User Manual
4535 616 38521
Use of the product for purposes other than those intended and expressly stated
by Philips, as well as incorrect use or operation, may relieve Philips or its agents
from all or some responsibility for resultant noncompliance, damage, or injury.
WARNING
System users are responsible for image quality and diagnosis.
Warnings
Before using the system, read these warnings and the «Safety» section.
WARNINGS
• Do not remove the protective covers on the system; hazardous voltages
are present inside. Cabinet panels must be in place while the system is in
use. All internal adjustments and replacements must be made by a qualified
Philips Ultrasound field service engineer.
• To avoid electrical shock, use only supplied power cords and connect only
to properly grounded wall (wall/mains) outlets.
• Do not operate the system in the presence of flammable anesthetics or
other flammable gases or liquids. Explosion can result.
• Medical equipment must be installed and put into service according to the
special electromagnetic compatibility (EMC) guidelines provided in the
«Safety» section.
• The use of portable and mobile radio-frequency (RF) communications
equipment can affect the operation of medical equipment.
CX50 User Manual
16
4535 616 38521
Read This First
1
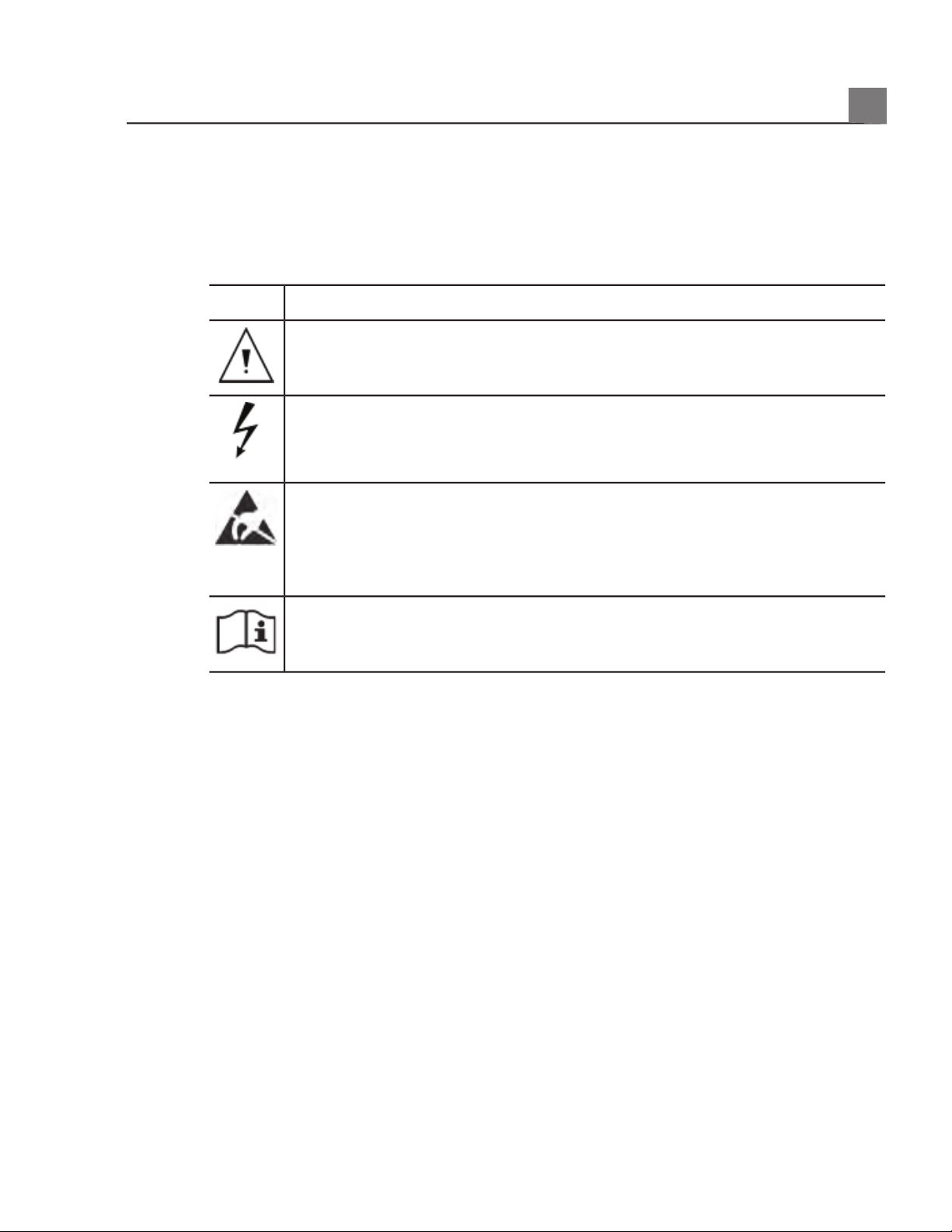
Warning Symbols
The system may use the following warning symbols. For additional symbols used
on the system, see the
«Safety» section.
DescriptionSymbol
Identifies a safety note.
Dangerous voltages: Appears adjacent to high-voltage terminals,
indicating the presence of voltages greater than 1,000 Vac (600 Vac in
the United States).
Identifies ESD (electrostatic-discharge) sensitivity of a connector that
is not tested as specified in IEC 60601-1-2. Do not touch exposed
connector pins. Touching exposed pins can cause electrostatic
discharge, which can damage the product.
Indicates that the user should see the instructions for use for safety
information.
User Information Components
The user information provided with your product includes the following
components:
• Compact Disc (CD): Includes all of the user information, except the
Operating Notes. The instructions for using the CD are included with the
CD.
• Operating Notes: Contains information that clarifies certain product
responses that might be misunderstood or cause user difficulty.
• User Manual: Provided with the product and included on the CD. The
User Manual introduces you to features and concepts, helps you set up your
system, and includes important safety information. This manual also includes
17
CX50 User Manual
4535 616 38521
1
Read This First

procedures for basic operation. For detailed operating instructions, see the
Help.
• CX50 Integrated Ultrasound User Manual: This manual introduces you
to Integrated Ultrasound, helps you set it up, and provides basic operating
instructions. For more information on the use and operation of your Allura
XPer FD system with Integrated Ultrasound, see the Allura XPer FD
instructions for use.
• Help: Available on the system in some languages and included on the CD,
the Help contains comprehensive instructions for using the system. The
Help also provides reference information and descriptions of all controls
and display elements. To display the Help, press Help on the system
keyboard.
• Acoustic Output Tables: Included on the CD, it contains information
about acoustic output and patient-applied part temperatures.
• Medical Ultrasound Safety: Included on the CD, it contains information
on bioeffects and biophysics, prudent use, and implementing ALARA (as
low as reasonably achievable).
• Shared Roles for System and Data Security: Included on the CD, it
contains guidelines to help you understand how the security of your Philips
product could be compromised and information on Philips’ efforts to help
you prevent security breaches.
• Media Compatibility: Included on the CD, it contains current information
on media that are compatible with your system.
Product Conventions
Your Philips product uses certain conventions throughout the interface to make
it easy for you to learn and use:
• Two unlabeled buttons, referred to as «trackball buttons,» are used with
the trackball. Those controls, located on either side of the trackball, operate
CX50 User Manual
18
4535 616 38521
Read This First
1

somewhat similarly to PC mouse buttons. Both trackball buttons function
identically.
• In the system setups, tabs along the top of the monitor display let you
choose additional sets of setup options.
• To type text into a text field, click in the field and use the keyboard.
•
To display a list, click the down arrow
. To scroll through a list, click the
arrows at either end of the scroll bar or drag the scroll box up or down.
• Controls on the control panel include buttons, knobs, slide controls, and
a trackball. Press a button to activate or deactivate its function. Turn a knob
to change the selected setting. Move a slide control to change its setting.
Roll the trackball in the direction that you want to move an object. The
current trackball function is displayed in the trackball select menu at the
bottom of the display.
• Controls across the top of the control panel, called quick keys, function as
both buttons and knobs. To select one of the functions displayed above the
control, simply press the control. To select a setting for the function, also
displayed above the control, turn the control.
User Information Conventions
The user information for your product uses the following typographical
conventions to assist you in finding and understanding information:
• All procedures are numbered, and all subprocedures are lettered. You must
complete steps in the sequence they are presented to ensure success.
• Bulleted lists indicate general information about a particular function or
procedure. They do not imply a sequential procedure.
• Control names and menu items or titles are spelled as they are on the
system, and they appear in bold text. The only exceptions are the trackball
and the buttons adjacent to it, which are unlabeled.
• Symbols appear as they appear on the system.
• The pointer is the cursor used to select elements on the display. Use the
Pointer control to display the pointer.
• Point means to position the tip of the pointer or cursor on an item on the
display.
19
CX50 User Manual
4535 616 38521
1
Read This First

• Click means to move the pointer to an object and press the left trackball
button.
• Select means to click a check box to put a check mark in it. Deselect means
clicking the check box to remove the check mark.
• Double-click means to quickly click twice to select an object or text.
• Right-click means to point at an item and then press and immediately release
the right trackball button.
• Hover means to pause the pointer over an item on the display.
• Drag means to place the pointer over an object and then press and hold
the left trackball button while moving the trackball. Use this method to
move an object on the display.
• Highlight means to change the color of a display selection (such as an item
in a list) or overlay it with a colored bar, usually by clicking.
• The left side of the system is to your left as you stand in front of the system,
facing the system. The front of the system is nearest to you as you operate
it.
• Transducers and pencil probes both are referred to as transducers, unless
the distinction is important to the meaning of the text.
Information that is essential for the safe and effective use of your product appears
throughout your user information as follows:
WARNING
Warnings highlight information vital to the safety of you, the operator, and the
patient.
CAUTION
Cautions highlight ways that you could damage the product and consequently
void your warranty or service contract or ways that you could lose patient or
system data.
NOTE
Notes bring your attention to important information that will help you operate
the product more effectively.
CX50 User Manual
20
4535 616 38521
Read This First
1

Upgrades and Updates
Philips is committed to innovation and continued improvement. Upgrades may
be announced that consist of hardware or software improvements. Updated
user information will accompany those upgrades.
Customer Comments
If you have questions about the user information, or you discover an error in
the user information, in the USA, please call Philips at 800-722-9377; outside
the USA, please call your local customer service representative.
Supplies and Accessories
To order ECG trunk cables, lead sets, and electrodes; transducer covers; bite
guards; biopsy guides; and other supplies and accessories, contact CIVCO Medical
Solutions:
CIVCO Medical Solutions
102 First Street South, Kalona, IA 52247-9589
Telephone: 800-445-6741 (USA and Canada), +1 319-248-6757 (International)
Fax: 877-329-2482 (USA and Canada), +1 319-248-6660 (International)
E-mail: info@civco.com
Internet: www.civco.com
To order the items listed in the following table, see the referenced section and
then contact your Philips representative.
21
CX50 User Manual
4535 616 38521
1
Read This First
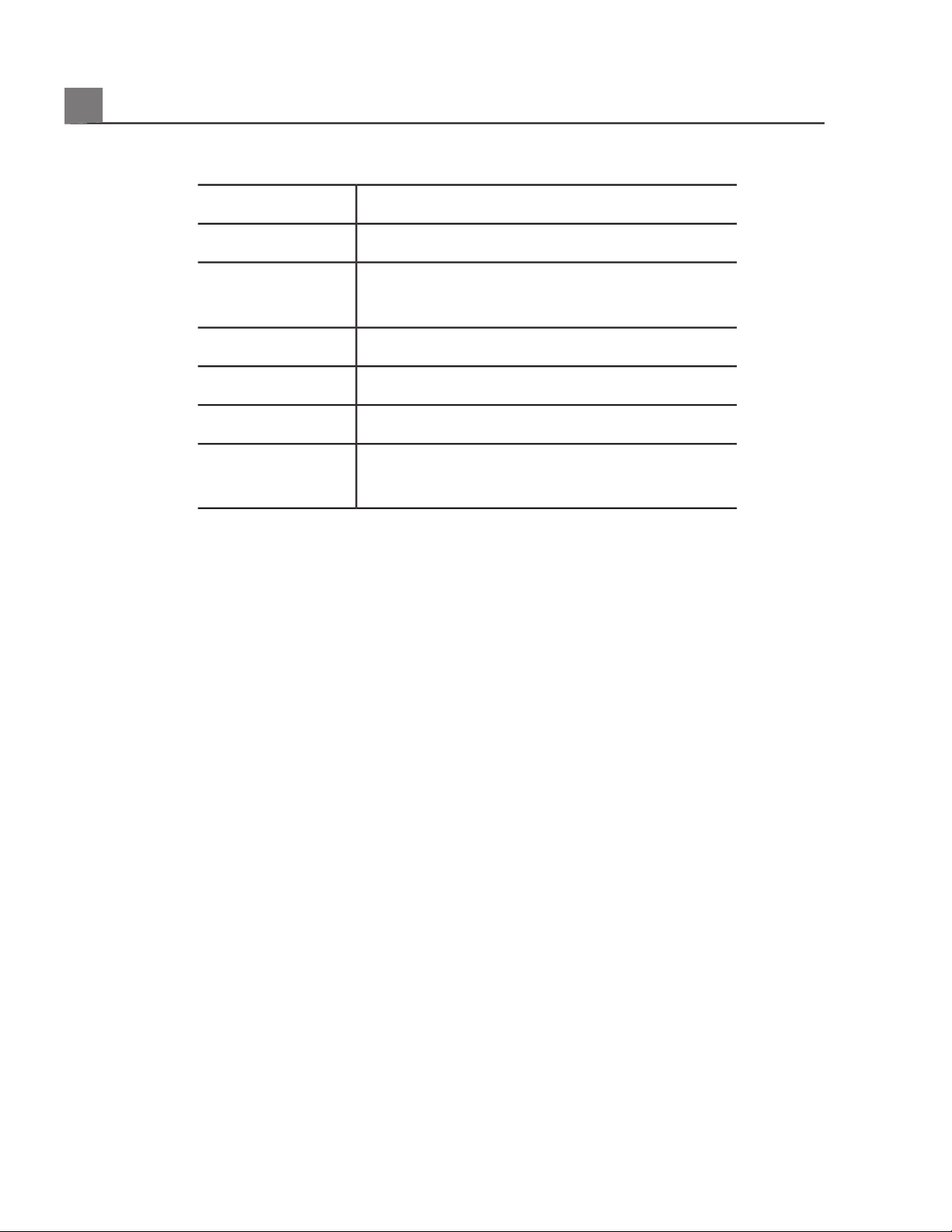
System Accessories
Additional InformationItem
Contact your Philips representative.Battery
See
«Approved Cables for Electromagnetic
Compliance» on page 70
Cables
Contact your Philips representative.Foot switch
See «External Printers» on page 100Printers
See
«Media Compatibility» on page 164Removable media
See
«Clinical Options and Transducers» on
page 195
Transducers
Customer Service
Customer service representatives are available worldwide to answer questions
and to provide maintenance and service. Please contact your local Philips
representative for assistance. You can also contact the following office for referral
to a customer service representative, or visit the Philips Healthcare «Contact
Us» website:
www.healthcare.philips.com/main/about/officelocator/index.wpd
Philips Ultrasound Headquarters
22100 Bothell-Everett Highway, Bothell, WA 98021-8431, USA
800-722-9377
Recycling, Reuse, and Disposal
Philips is concerned with helping protect the natural environment and helping
ensure continued safe and effective use of this system through proper support,
maintenance, and training. Philips designs and manufactures equipment in
compliance with relevant guidelines for environmental protection. As long as
CX50 User Manual
22
4535 616 38521
Read This First
1

the equipment is properly operated and maintained, it presents no risk to the
environment. However, the equipment may contain materials that could be
harmful to the environment if disposed of incorrectly. Use of such materials is
essential for the implementation of certain functions and for meeting certain
statutory and other requirements.
The European Union Directive on Waste Electrical and Electronic Equipment
(WEEE) requires producers of electrical and electronic equipment to provide
reuse and treatment information for each product. This information is provided
in a Philips Healthcare Recycling Passport. Such recycling passports for Philips
Ultrasound systems are available on this website:
www.healthcare.philips.com/main/about/sustainability/recycling/ultrasound.wpd
Recycling, reuse, and disposal information in this document is directed mainly at
the entity with legal authority over the equipment. Operators are usually
uninvolved in disposal, except in the case of certain batteries (see
«Battery
Operation» on page 148
).
Passing Your System to Another User
If you pass this system to another user who will use the system for its intended
purpose, then pass it on in its complete state. Particularly, ensure that all the
product-support documentation, including all instructions for use, are passed on
to the new user. Make the new user aware of the support services that Philips
Healthcare provides for installing, commissioning, and maintaining the system,
and for comprehensive operator training. Existing users must remember that
passing on medical electrical equipment to new users may present serious
technical, medical, privacy, and legal risks. The original user may remain liable,
even if the equipment is given away.
Philips strongly advises you to seek advice from your local Philips representative
before agreeing to pass on any equipment.
After you pass the system to a new user, you might still receive important
safety-related information, such as bulletins and field change orders. In many
jurisdictions the original owner has a clear duty to communicate such
safety-related information to new users. If you are unable or unprepared to do
23
CX50 User Manual
4535 616 38521
1
Read This First
this, inform Philips Healthcare about the new user, so that Philips Healthcare
can provide the new user with safety-related information.
Final Disposal of Your System
Final disposal is when you dispose of the system in such a way that it can no
longer be used for its intended purposes.
WARNING
Do not dispose of this system (or any parts of it) with industrial or domestic
waste. The system may contain materials such as lead, tungsten, or oil, or other
hazardous substances that can cause serious environmental pollution. The system
also contains privacy-sensitive information, which should be properly removed
(scrubbed). Philips advises you to contact your Philips service organization before
disposing of this system.
Philips Healthcare gives support for the following:
• Recovery of useful parts
• Recycling of useful materials by competent disposal companies
• Safe and effective disposal of equipment
For advice and information, contact your Philips service organization, or see the
following website:
www.philips.com/about/sustainability/recycling/productrecyclingservices/index.page
Perchlorate Material
In this system, perchlorate material is present in lithium coin cells or batteries.
Special handling may apply to those items. For more information, see this website:
www.dtsc.ca.gov/hazardouswaste/perchlorate
CX50 User Manual
24
4535 616 38521
Read This First
1
2 Safety
Please read this information before using your ultrasound system. It applies to
the ultrasound system, transducers, recording devices, and any optional
equipment. This section covers general safety information only. Safety
information that applies only to a specific task is included in the procedure for
that task.
This device is intended for use by, or by the order of, and under the supervision
of a licensed physician qualified to direct the use of the device.
A WARNING describes precautions necessary to prevent injury or loss of
life.
A CAUTION describes precautions necessary to protect the equipment and
patient or system data.
Basic Safety
WARNINGS
• Do not use the system for any application until you have read, understood,
and know all the safety information, safety procedures, and emergency
procedures contained in this «Safety» section. Operating the system
without a proper awareness of safe use could lead to fatal or other serious
personal injury.
• Do not use this system for any application until you are sure that the
system’s periodic maintenance is current. If any part of the system is
known or suspected to be defective or incorrectly adjusted, do not use
the system until it is repaired. Operating the system with defective or
incorrectly adjusted components could expose you and the patient to
safety hazards.
• Do not use the system for any application until you are adequately and
properly trained on its safe and effective operation. If you are unsure of
your ability to operate the system safely and effectively, do not use it.
25
CX50 User Manual
4535 616 38521
Operation of the system without proper and adequate training could lead
to fatal or other serious personal injury.
• Do not operate the system with patients unless you have an adequate
understanding of its capabilities and functions. Using the system without
such understanding may compromise the system’s effectiveness and the
safety of the patient, you, and others.
• Never attempt to remove, modify, override, or frustrate any safety device
on the system. Interfering with safety devices could lead to fatal or other
serious personal injury.
• Use the system only for its intended purposes. Do not use the system with
any product that Philips does not recognize as compatible with the system.
Operation of the product for unintended purposes, or with incompatible
products, could lead to fatal or other serious injury.
Electrical Safety
This equipment has been verified by a recognized third-party testing agency as
a Class I device with Type BF and Type CF isolated patient-applied parts, and
Type B non-isolated patient-applied parts. (The safety standards met by this
system are included in the
«Specifications» section.) For maximum safety observe
these warnings and cautions:
WARNINGS
• Shock hazards may exist if this system (when mounted on its cart or plugged
directly into an AC power source), including all externally mounted
recording and monitoring devices, is not properly grounded. Protection
against electrical shock is provided by grounding the cart or the AC power
adapter with a three-wire cable and plug, which must be plugged into a
grounded outlet. The grounding wire must not be removed or defeated.
• To avoid the risk of electrical shock, never connect the system power cord
to a power strip or an extension cord. When using the power cord, always
connect it directly to a grounded wall outlet.
• Use only the AC adapter supplied with your system.
CX50 User Manual
26
4535 616 38521
Safety
2

• Use only Type CF transducers for invasive procedures. Type B transducers
are insufficiently electrically isolated for invasive use.
• Do not remove the protective covers on the system; hazardous voltages
are present inside. Cabinet panels must be in place while the system is in
use. All internal adjustments and replacements must be made by a qualified
Philips Ultrasound field service engineer.
• Do not operate this system in the presence of flammable gases or
anesthetics. Explosion can result. The system is not compliant in AP/APG
environments as defined by IEC 60601-1.
• To avoid risk of electrical shock hazards, always inspect the transducer
before use: Check the face, housing, and cable before use. Do not use if
the face is cracked, chipped, or torn; the housing is damaged; or the cable
is abraded.
• To avoid risk of electrical shock hazards, always turn off the system,
disconnect it from the wall outlet, and remove the battery (see
«Installing
the Battery» on page 149
) before cleaning the system.
• All patient-contact devices, such as transducers, pencil probes, and ECG
leads not specifically indicated as defibrillation-proof must be removed from
patient contact before application of a high-voltage defibrillation pulse. See
«Defibrillators» on page 29.
• During transesophageal echocardiographic (TEE) procedures, either remove
the TEE transducer from the patient or disconnect the TEE transducer from
the system immediately following image acquisition.
• Ultrasound equipment in normal operation, as with other medical electronic
diagnostic equipment, uses high-frequency electrical signals that can interfere
with pacemaker operation. Though the possibility of interference is slight,
be alert to this potential hazard and stop system operation immediately if
you note interference with a pacemaker.
• When using additional peripheral equipment powered from an electrical
source other than the ultrasound system, the combination is considered
to be a medical system. It is your responsibility to comply with
IEC 60601-1-1 and test the system to those requirements. If you have
questions, contact your Philips representative.
• Do not use nonmedical peripherals, such as report printers, within 1.5 m
(5 ft) of a patient, unless the nonmedical peripherals receive power from
27
CX50 User Manual
4535 616 38521
2
Safety

an isolated power outlet on the Philips ultrasound system, or from an
isolation transformer that meets medical safety standards, as defined by
standard IEC 60601-1-1.
• The system and patient-applied parts meet the standard IEC 60601-1.
Applied voltages exceeding the standard, although unlikely, may result in
electrical shock to the patient or operator.
• Connection of optional devices not supplied by Philips Ultrasound could
result in electrical shock. When such optional devices are connected to
your ultrasound system, ensure that the total system earth leakage current
does not exceed 500 µA, or in the United States, 300 µA.
• To avoid risk of electrical shock, do not use any transducer that has been
immersed beyond the specified cleaning or disinfection level.
• To avoid risks of electrical shock and fire hazards, inspect the system power
cord and plug regularly. Ensure that they are not damaged in any way.
• Do not drape the power cord over any of the cable hooks or the handle
on the system cart. Damage to the cord or power receptacle unit can occur
if the cart is raised.
• Operating the system with physio input signals that are below the specified
minimum levels may cause inaccurate results. See the
«Specifications» section.
• Electrosurgical units (ESUs) and other devices intentionally introduce radio
frequency electromagnetic fields or currents into patients. Because imaging
ultrasound frequencies are coincidentally in the radio frequency range,
ultrasound transducer circuits are susceptible to radio frequency
interference. While an ESU is in use, severe noise interferes with the
black-and-white image and completely obliterates the color image.
Concurrent failures in an ESU or other device and in the outer layer of the
TEE transducer shaft can cause electrosurgical currents to return along the
transducer conductors. This could burn the patient, and the ultrasound
system and the transducer could also be damaged. Be aware that a disposable
transducer cover provides no protective electrical insulation at ESU
frequencies.
• To avoid risk of a burn hazard, do not use transducers with high-frequency
surgical equipment. A burn hazard may result from a defect in the
high-frequency surgical neutral electrode connection.
CX50 User Manual
28
4535 616 38521
Safety
2
• Using cables, transducers, and accessories other than those specified for
use with the system may result in increased emissions from, or decreased
immunity of, the system.
CAUTIONS
• Although your system has been manufactured in compliance with existing
EMI/EMC requirements, use of this system in the presence of an
electromagnetic field can cause momentary degradation of the ultrasound
image. When interference is present or intermittent, use caution when
continuing to use the system. If interference occurs often, review the
environment in which the system is being used, to identify possible sources
of radiated emissions. These emissions could be from other electrical devices
used within the same room or an adjacent room. Communication devices
such as cellular phones and pagers can cause these emissions. The existence
of radio, TV, or microwave transmission equipment located nearby can
cause emissions. In cases where EMI is causing disturbances, it may be
necessary to relocate your system.
• For information on electromagnetic emissions and immunity as it applies
to the system, see
«Electromagnetic Compatibility» on page 66. Ensure that
the
operating environment of your system meets the conditions specified
in the referenced information. Operating the system in an environment that
does not meet those conditions may degrade system performance.
Defibrillators
Observe the following warnings when a defibrillation is required while using the
ultrasound system.
WARNINGS
• Before defibrillation, always remove all patient-applied parts from the patient.
• Before defibrillation, always disconnect invasive transducers from the system.
• A disposable transducer cover provides no protective electrical insulation
against defibrillation.
• A small hole in the outer layer of the transducer opens a conductive path
to grounded metal parts of the transducer. The secondary arcing that could
29
CX50 User Manual
4535 616 38521
2
Safety
occur during defibrillation could cause patient burns. The risk of burns is
reduced, but not eliminated, by using an ungrounded defibrillator.
Use defibrillators that do not have grounded patient circuits. To determine
whether a defibrillator patient circuit is grounded, see the defibrillator service
guide, or consult a biomedical engineer.
Fire Safety
WARNING
On electrical or chemical fires, use only extinguishers that are specifically labeled
for those purposes. Using water or other liquids on an electrical fire can lead to
fatal or other serious personal injury. Before attempting to fight a fire, if it is safe
to do so, attempt to isolate the product from electrical and other supplies, to
reduce the risk of electrical shock.
Use of electrical products in an environment for which they were not designed
can lead to fire or explosion. Fire regulations for the type of medical area being
used should be fully applied, observed, and enforced. Fire extinguishers should
be available for both electrical and nonelectrical fires.
Mechanical Safety
A list of precautions related to mechanical safety follows; observe these
precautions when using the system:
WARNINGS
• Be aware of the wheels on the system cart, especially when moving the
system. The system could cause injury to you or others if it rolls over feet
or into shins. Use caution when going up or down ramps.
• When attempting to overcome an obstacle, do not push the system from
either side with excessive force, which could cause the system to tip over.
• Position external hardcopy devices away from the system. Ensure that they
are secure. Do not stack them on the system.
CX50 User Manual
30
4535 616 38521
Safety
2

• When positioning the monitor, move it carefully to avoid pinching hands
or extremities against other objects, such as a bed rail.
• Never park the system on an incline.
• The brakes are intended as a convenience. To increase cart security, use
wheel chocks when the system is parked.
• If system operation is abnormal after you move or transport the system,
contact Philips Ultrasound Customer Service immediately. System
components are installed securely and can withstand considerable shock,
but excessive shock can cause a system failure.
• To avoid injury, Philips recommends against lifting the system cart.
CAUTIONS
• Before moving the system, ensure that the system is secured for transport.
On some systems, that may include ensuring that the monitor is latched,
to prevent monitor damage during transport.
• Ensure that the cables for all patient-applied parts are secure before moving
the system. Use the cable management system to ensure that transducer
cables are protected from damage.
• Do not roll the system over transducer cables or power cables.
• Do not use the system handle or transducer holders to move the cart.
• Never move the cart with the system on it, unless the system is properly
attached to the cart.
• To avoid the possibility of tipping the system cart when you move it over
a threshold, lift the cart slightly with the handle on the rear of the cart and
pull the cart over the threshold.
Equipment Protection
Follow these precautions to protect your system:
31
CX50 User Manual
4535 616 38521
2
Safety

CAUTIONS
• Excessive bending or twisting of cables on patient-applied parts may cause
failure or intermittent operation of the system. Do not roll the system over
cables, which may damage them.
• Improper cleaning or sterilization of a patient-applied part may cause
permanent damage. For cleaning and disinfection instructions, see the
«Transducer Care» section.
• Do not submerge the transducer connector in solution. The cables and
transducer bodies are liquid-tight, but the connectors are not.
• Do not use solvents, such as thinner or acetone, or abrasive cleaners on
the system, transducers, or any hardcopy device.
• For optimal performance, connect your ultrasound system to a circuit
dedicated solely for the system. Do not connect life-support devices to the
same circuit as the ultrasound system.
• If systems, transducers, and peripherals have been in an environment below
10°C (50°F), allow them to reach room temperature before connecting or
turning them on. Philips recommends allowing 24 hours for complete
normalization. Otherwise, condensation inside the devices could cause
damage. If the device was only briefly exposed to temperatures below 10°C
(50°F), then the time required for the device to return to room temperature
could be significantly less than 24 hours.
• To avoid damaging the flat-panel display in the monitor, do not store the
system where the ambient temperature exceeds 65°C (149°F).
Product Compatibility
Do not use your system in combination with other products or components,
unless Philips expressly recognizes those other products or components as
compatible. For information about such products and components, contact your
Philips representative.
Changes and additions to the system should be made only by Philips or by third
parties expressly authorized by Philips to do so. Such changes and additions must
CX50 User Manual
32
4535 616 38521
Safety
2
comply with all applicable laws and regulations that have the force of law within
the jurisdictions concerned, and best engineering practices.
WARNING
System changes and additions that are made without the appropriate training or
by using unapproved spare parts may void the Philips warranty. As with all
complex technical products, maintenance by unqualified persons or using
unapproved spare parts carries serious risks of system damage and personal
injury.
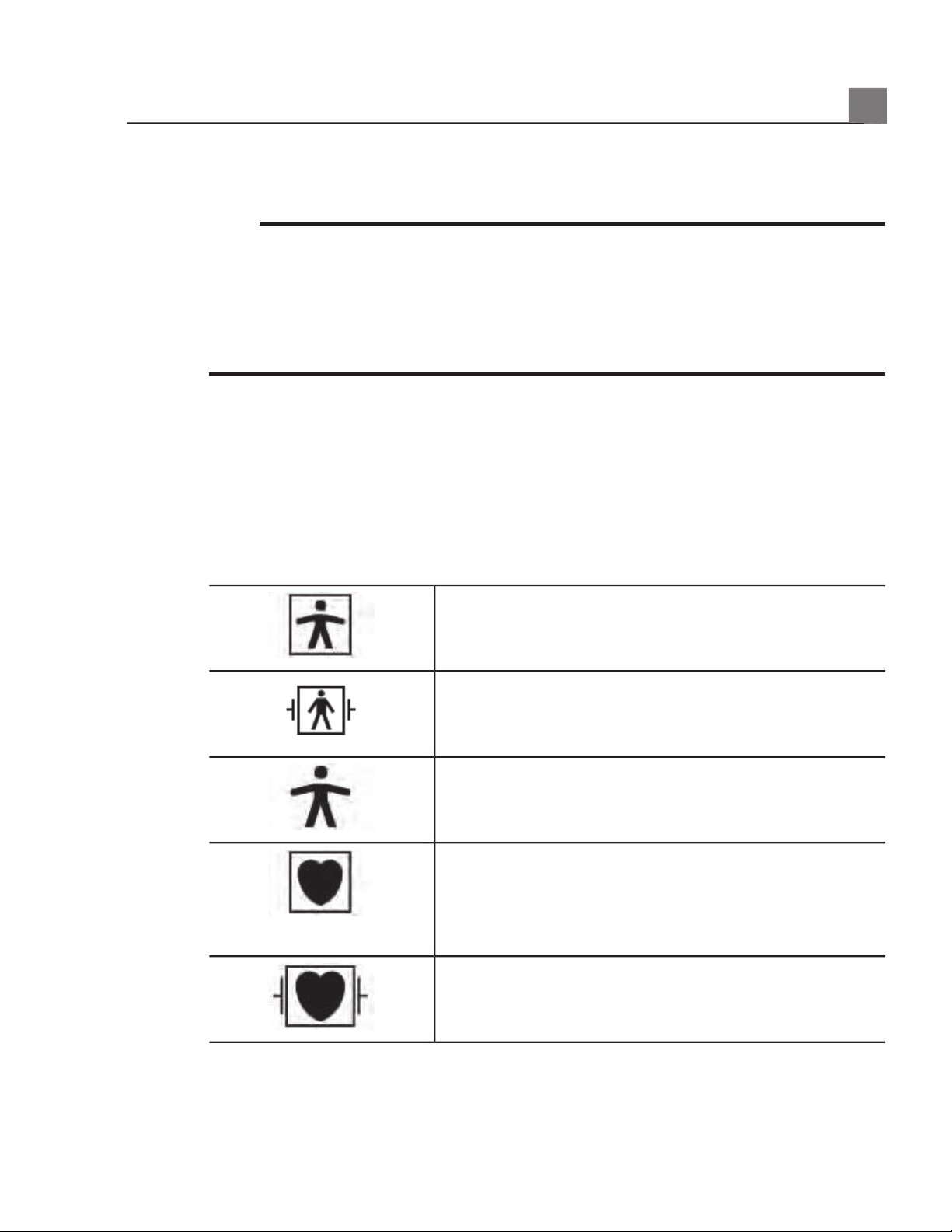
Symbols
The International Electrotechnical Commission (IEC) has established a set of
symbols for medical electronic equipment that classify a connection or warn of
potential hazards. Of those symbols, the following may be used on your Philips
product and its accessories and packaging.
Isolated patient connection (Type BF applied part).
Defibrillation-proof patient connection (Type BF
applied part).
Non-isolated patient connection (Type B applied
part).
Isolated patient connection for applied part intended
for intraoperative use, including direct cardiac
application and contact with major vessels (Type CF
applied part).
Defibrillation-proof patient connection (Type CF
applied part).
33
CX50 User Manual
4535 616 38521
2
Safety
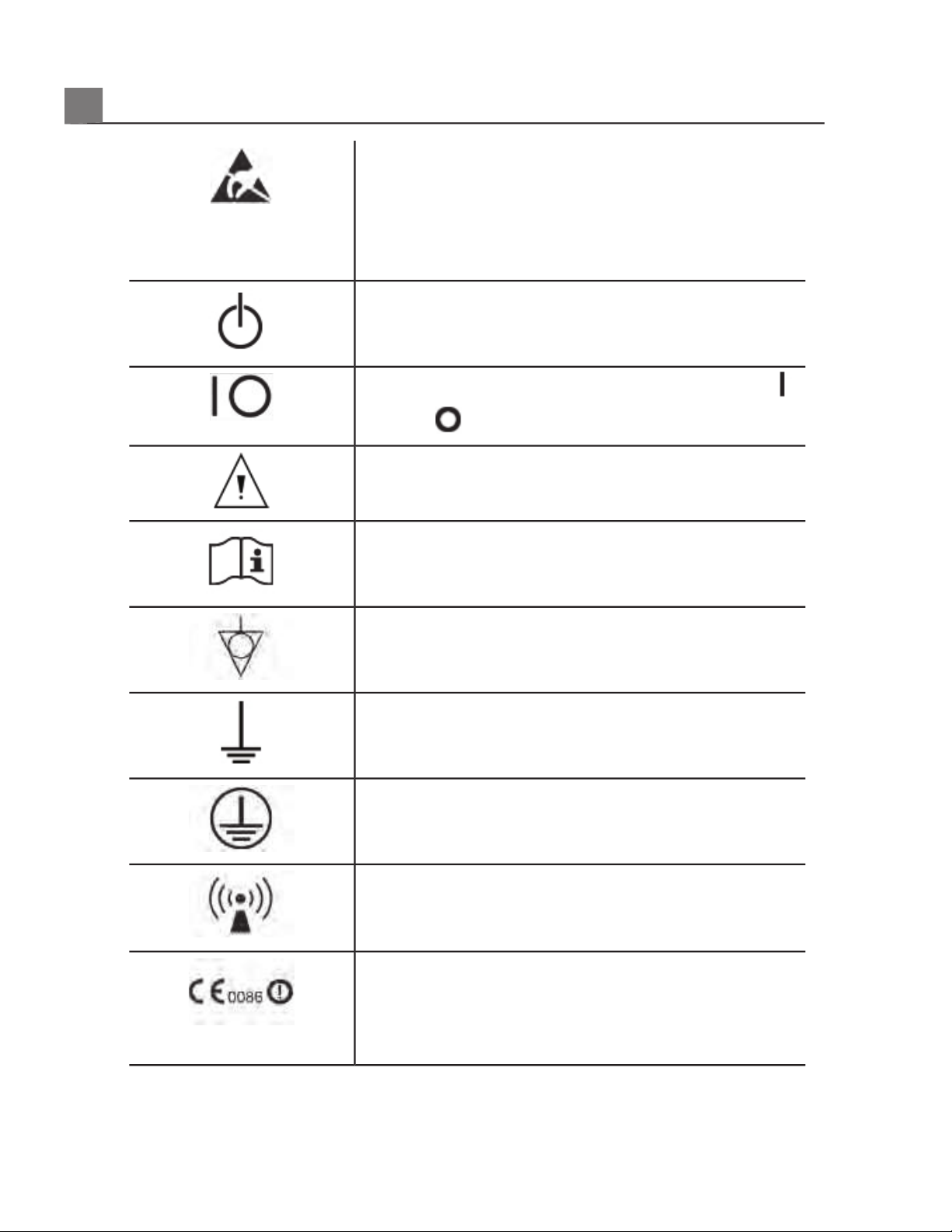
Identifies ESD (electrostatic-discharge) sensitivity of
a connector that is not tested as specified in
IEC 60601-1-2. Do not touch exposed connector
pins. Touching exposed pins can cause electrostatic
discharge, which can damage the product.
Identifies an On/Off control.
On a two-position power switch, represents On ( )
and Off (
).
Identifies a safety note.
Indicates that the user should see the instructions
for use for safety information.
Identifies equipotential ground.
Identifies earth ground.
Identifies protective earth ground.
Nonionizing electromagnetic radiation. Indicates that
interference may occur in the vicinity of equipment
marked with this symbol.
The radio component contained in this device is
compliant to Council Directive 1999/5/EC (Radio
Equipment and Telecommunications Terminal
Equipment Directive).
CX50 User Manual
34
4535 616 38521
Safety
2
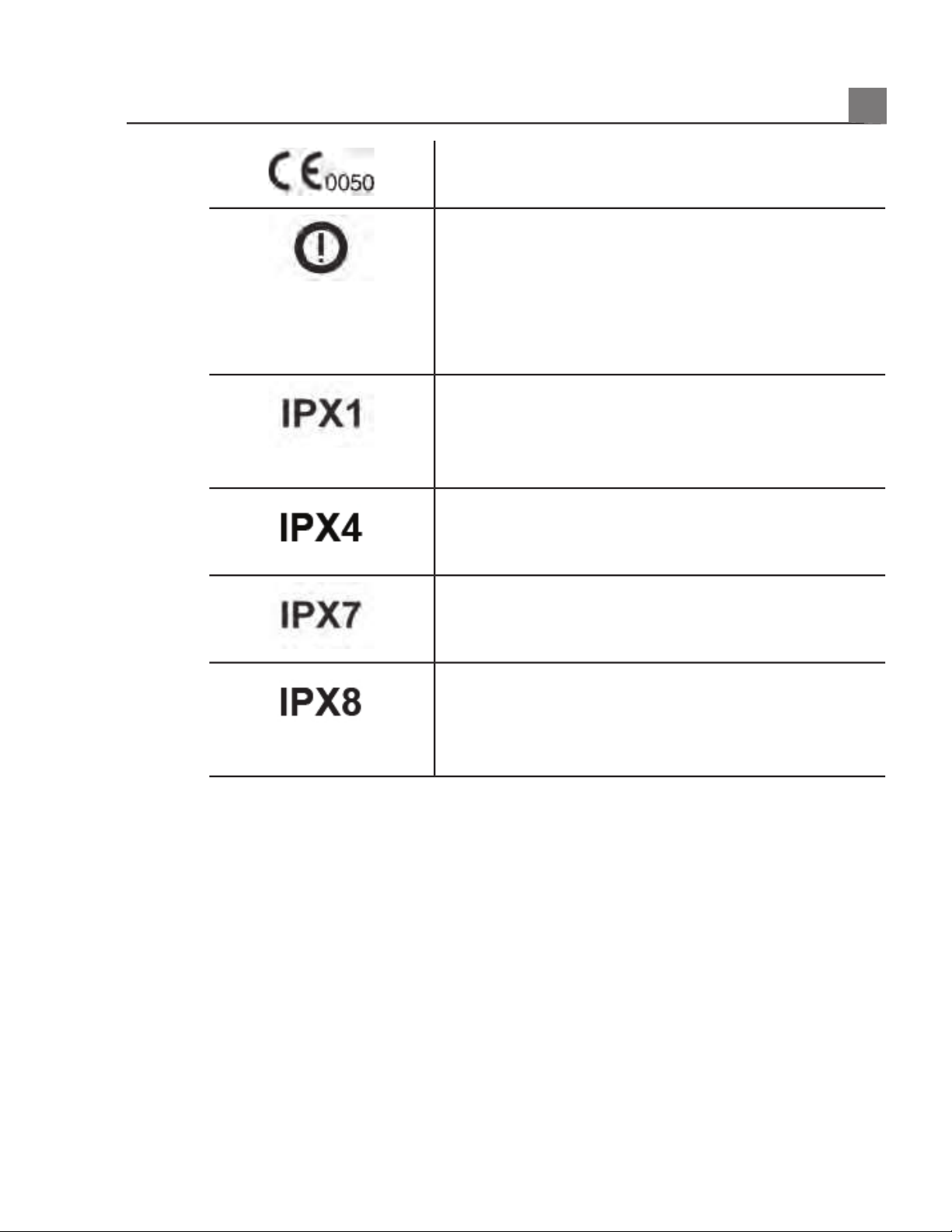
Indicates conformance with European Council
Directive 93/42/EEC.
Class 2 radio equipment identifier per Directive
1999/5/EC. European Union member states may apply
restrictions on putting this device into service or
placing it on the market. This device is intended to
be connected to the Publicly Available Interfaces for
use throughout the European Economic Area.
Indicates that the device is protected against the
effects of vertically falling water. This degree of
protection can apply to transducers or foot-operated
devices.
Indicates that the device is protected against the
effects of splashing liquids. This degree of protection
can apply to foot-operated devices.
Indicates that the device is protected against the
effects of immersion. This degree of protection can
apply to transducers and foot-operated devices.
Indicates that the device is protected against the
effects of immersion for up to 60 minutes. This
degree of protection can apply to foot-operated
devices.
35
CX50 User Manual
4535 616 38521
2
Safety
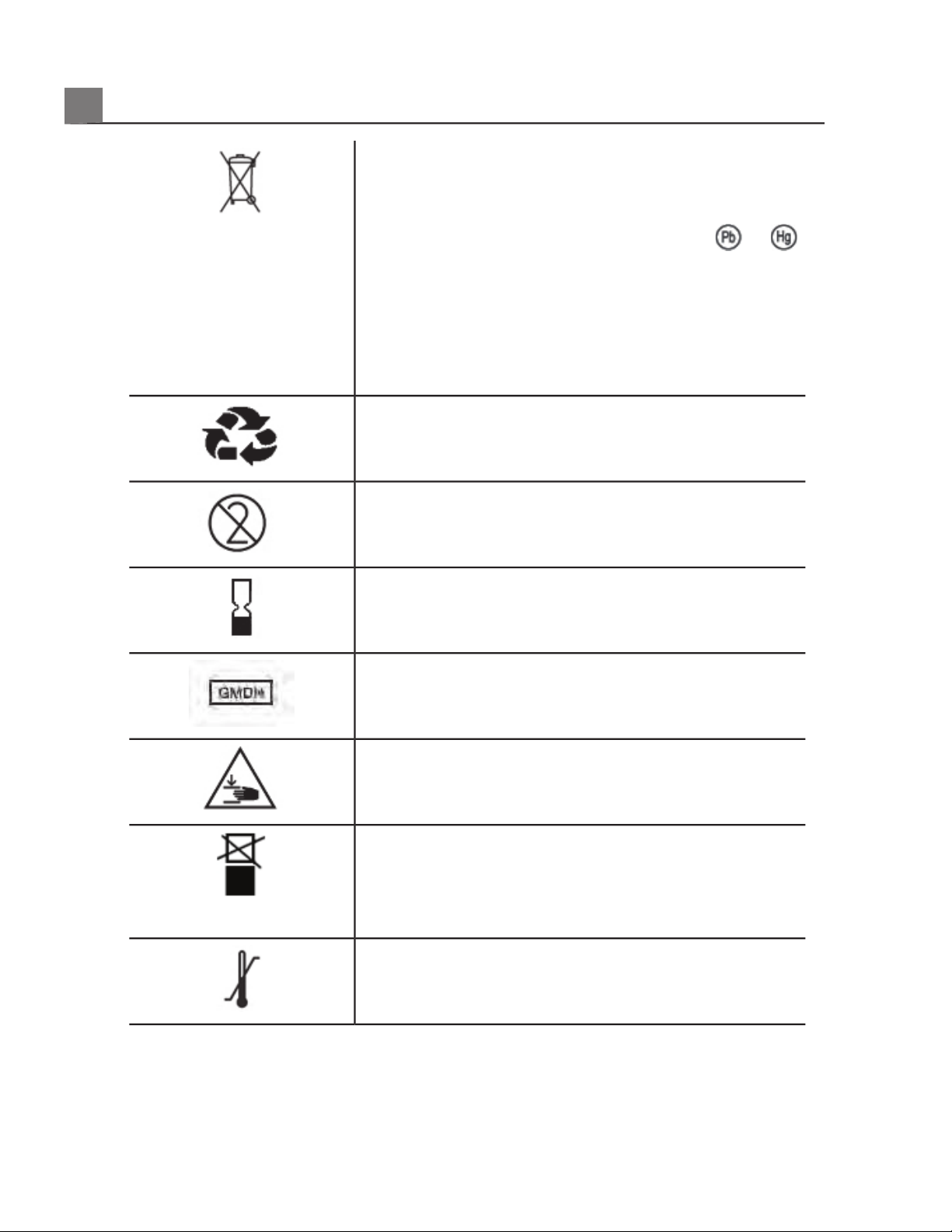
Indicates the need for separate collection for
electrical and electronic equipment in compliance
with the Waste Electrical and Electronic Equipment
(WEEE) Directive. When accompanied by
or ,
components of the device may contain lead or
mercury, respectively, which must be recycled or
disposed of in accordance with local, state, or federal
laws. The backlight lamps in an LCD system monitor
contain mercury.
Do not throw away. Dispose of in accordance with
local, state, or federal laws.
Do not reuse.
Use-by date.
Global Medical Device Nomenclature Code.
Indicates a possible crushing hazard to hands.
Warns that the system should not be used stacked
with other equipment. If the system is used stacked
with or adjacent to other equipment, verify normal
operation before use.
Indicates the temperature range (noncondensing) for
transport and storage. (Does not apply to media.)
CX50 User Manual
36
4535 616 38521
Safety
2
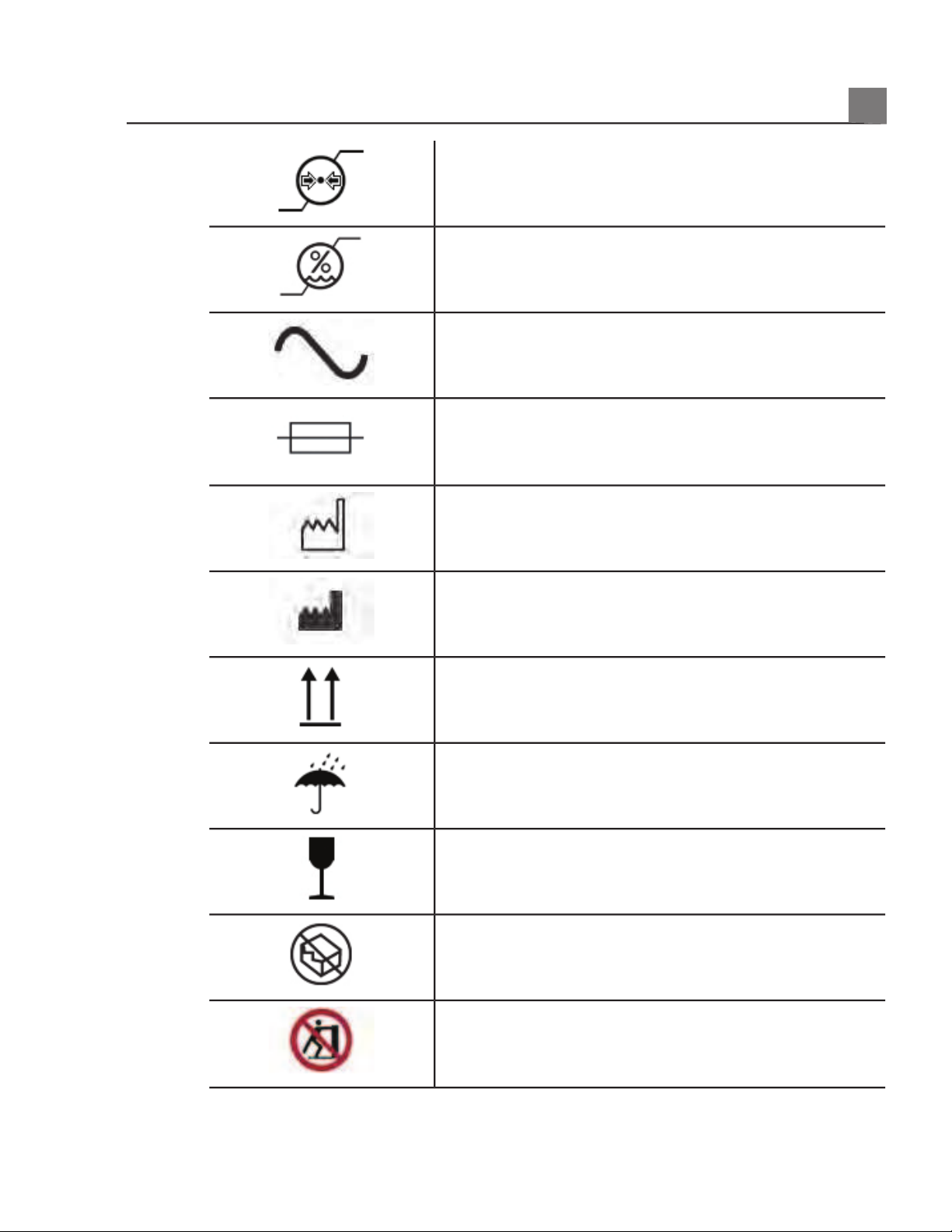
Indicates the atmospheric pressure range for
transport and storage.
Indicates the relative humidity range (noncondensing)
for transport and storage.
Indicates that a connector receives alternating
current.
Identifies fuse boxes or their locations. For continued
protection from fire and shock, replace fuses only
with fuses of the same type and rating.
Identifies the date of manufacture.
Identifies the legal manufacturer.
This side up: Points toward the side of the shipping
crate that should be kept facing up.
Indicates that the device should be kept dry.
Indicates that the device is fragile; handle with care.
Do not use if damaged.
Warns of system over-balance due to external force.
(Do not push on the monitor or the transducer
holders to move the system.)
37
CX50 User Manual
4535 616 38521
2
Safety
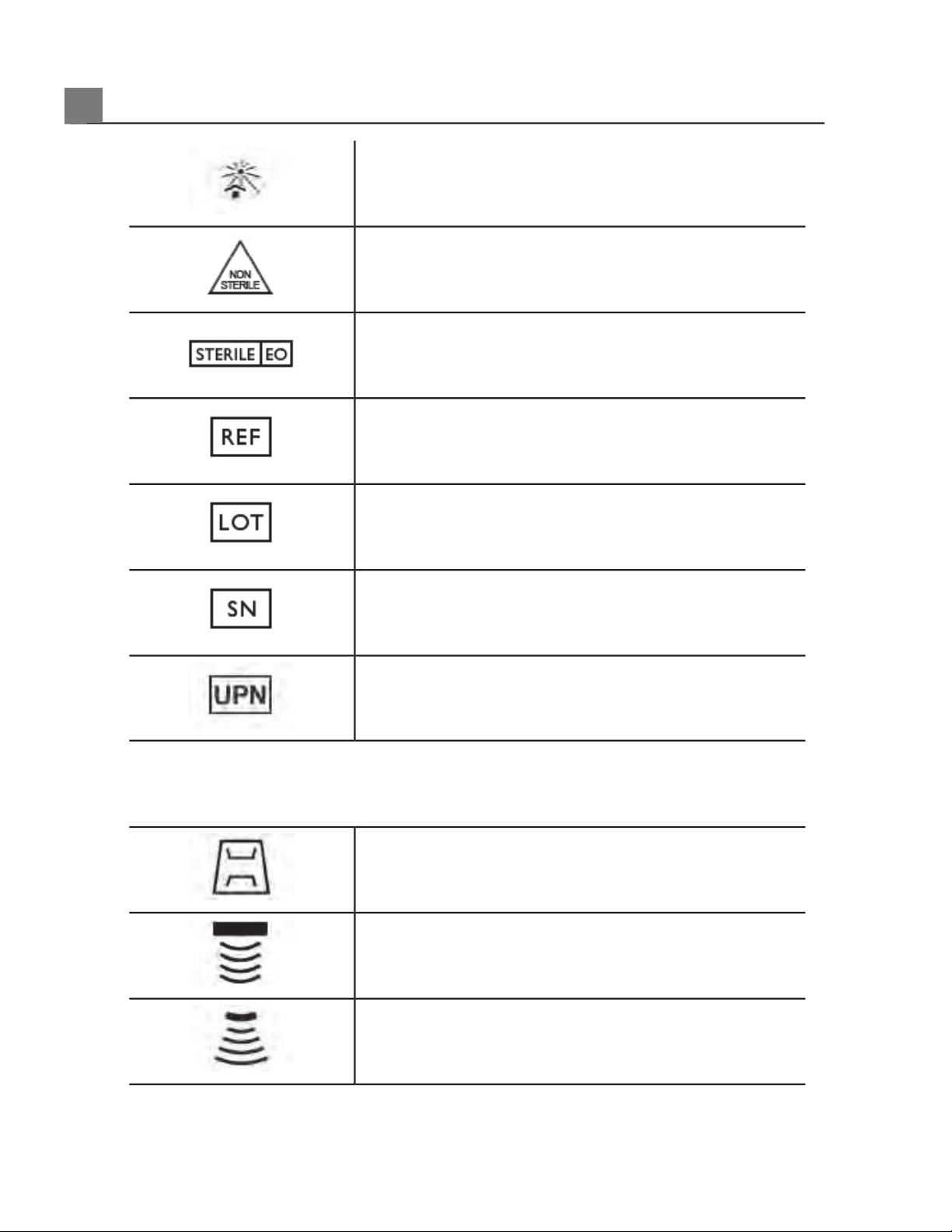
Keep away from sunlight.
Non-sterile.
Sterilized using ethylene oxide.
Catalog number.
Batch code.
Serial number.
Universal part number.
The following symbols may also be used on the system and its accessories and
packaging:
Connection for a pencil probe
Connection for a pencil probe
Connection for a transducer
CX50 User Manual
38
4535 616 38521
Safety
2
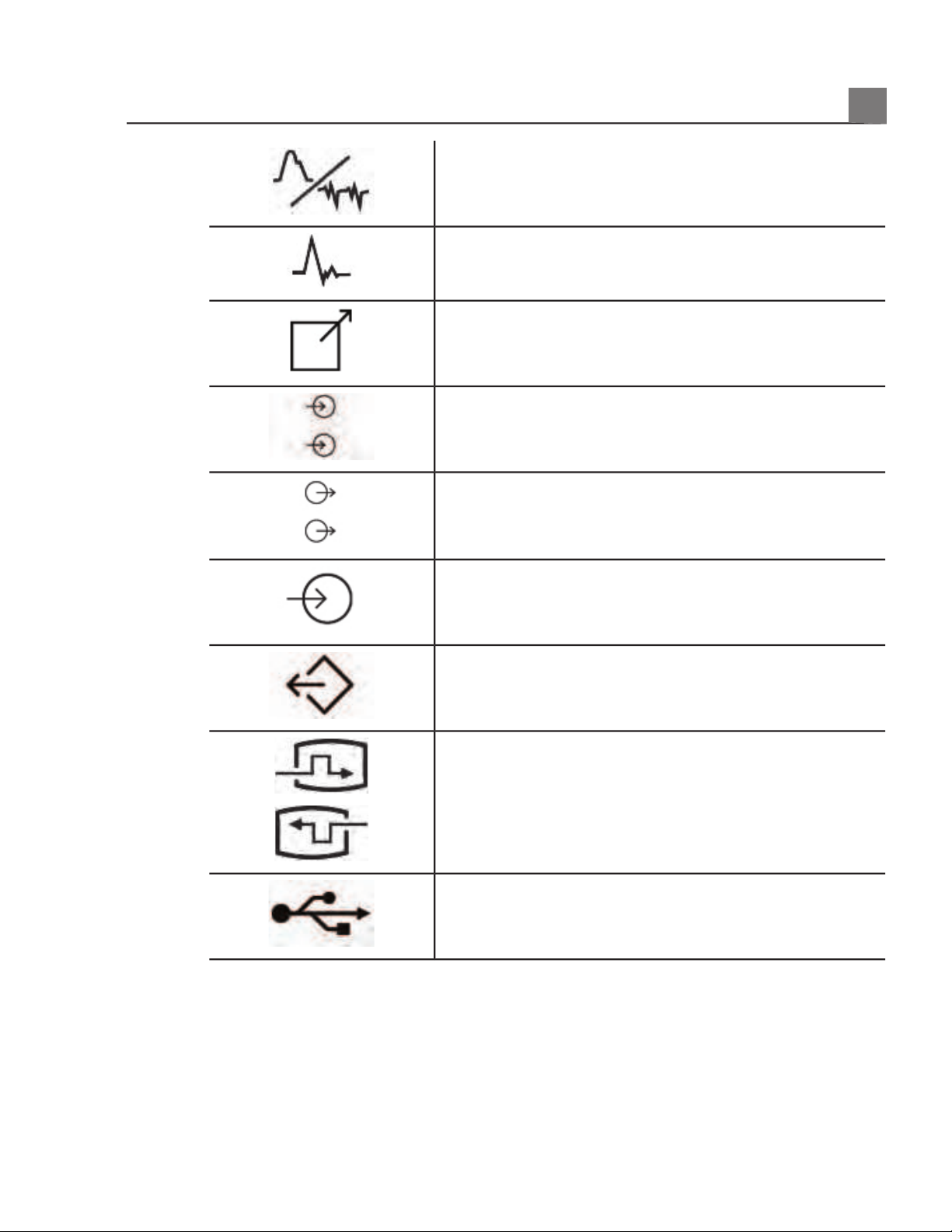
Connection for ECG leads
Connection for ECG leads
Print remote output
Input port for audio left/right, VHS/S-VHS,
microphone, CD, or DVD
Output port for audio left/right, VHS/S-VHS, video
patient monitor, black-and-white printer, or
interlaced RGB output port
Input port
VGA or parallel output port
DVI video output receptacle
USB input/output port
39
CX50 User Manual
4535 616 38521
2
Safety
FireWire (IEEE 1394) input/output port
Ethernet connection
RS-232 serial port
System microphone
Isolated auxiliary power provided for connection of
Philips-approved remote accessories.
Foot switch
Indicates the atmospheric pressure range for
transport and storage.
SVGA or DVI-I connection.
S-Video connection
CX50 User Manual
40
4535 616 38521
Safety
2
B/W Composite video output connection
Color composite video output connection
Video print trigger connection
Russian approval (GOST)
Identifies the port for the PercuNav tool connection
unit.
Identifies the port for the PercuNav field generator.
Chinese Environmentally Friendly Use Period symbol.
UL (Underwriters Laboratories) classification symbol.
CSA (CSA International) classification symbol.
Indicates a possible pinch hazard when positioning
the monitor.
41
CX50 User Manual
4535 616 38521
2
Safety
The following symbols may be used inside the system:
Dangerous voltages: Appears adjacent to high-voltage
terminals, indicating the presence of voltages greater
than 1,000 Vac (600 Vac in the United States).
Identifies equipotential ground.
Biological Safety
This section contains information about biological safety and a discussion of the
prudent use of the system.
A list of precautions related to biological safety follows; observe these precautions
when using the system. For more information refer to Medical Ultrasound Safety
on your user information CD.
WARNINGS
• Do not use the system if an error message on the video display indicates
that a hazardous condition exists. Note the error code, turn off power to
the system, and call your customer service representative.
• Do not use a system that exhibits erratic or inconsistent image updating.
Discontinuities in the scanning sequence indicate a hardware failure that
must be corrected before use.
• Perform ultrasound procedures prudently. Use the ALARA (as low as
reasonably achievable) principle.
• Use only acoustic standoffs that have been approved by Philips Ultrasound.
For information on ordering approved accessories, see
«Supplies and
Accessories» on page 21
.
• Verify the alignment of the biopsy guide before use. See the
«Biopsy Guides»
section.
• Verify the condition of the biopsy needle before use. Do not use a bent
biopsy needle.
CX50 User Manual
42
4535 616 38521
Safety
2

• Transducer covers may contain natural rubber latex. Those covers may
cause allergic reactions in some individuals. See
«FDA Medical Alert on
Latex» on page 44
.
• The M2203A bite guard strap contains natural rubber latex, which may
cause allergic reactions. See
«FDA Medical Alert on Latex» on page 44.
• In contrast studies using a high-MI acoustic field, capillary rupture, due to
microbubble expansion within a capillary in an acoustic field, can cause
extravasation. References: (1) Skyba, D.M., Price, R.J., Linka, A.Z., Skalak,
T.C., Kaul, S. «Direct in vivo visualization of intravascular destruction of
microbubbles by ultrasound and its local effects on tissue.» Circulation, 1998;
98:290-293. (2) van Der Wouw, P.A., Brauns, A.C., Bailey, S.E., Powers, J.E.,
Wilde, A.A. «Premature ventricular contractions during triggered imaging
with ultrasound contrast.» Journal of the American Society of Echocardiography,
2000;13(4):288-94.
• Preventricular contractions can be caused by the oscillations of microbubbles
when a high-MI acoustic field is triggered in the heart at the end of systole.
In a very sick patient with certain risk factors, theoretically, this could lead
to ventricular fibrillation. Reference: van Der Wouw, P.A., Brauns, A.C.,
Bailey, S.E., Powers, J.E., Wilde, A.A. «Premature ventricular contractions
during triggered imaging with ultrasound contrast.» Journal of the American
Society of Echocardiography, 2000;13(4):288-94.
• If a sterile transducer cover becomes compromised during an intraoperative
application involving a patient with transmissible spongiform encephalopathy,
such as Creutzfeldt-Jakob disease, follow the guidelines of the U.S. Centers
for Disease Control and this document from the World Health Organization:
WHO/CDS/ APH/2000/3, WHO Infection Control Guidelines for
Transmissible Spongiform Encephalopathies. The transducers for your
system cannot be decontaminated using a heat process.
• If the system becomes contaminated internally with bodily fluids carrying
pathogens, you must immediately notify your Philips service representative.
Components inside the system cannot be disinfected. In that case, the
system must be disposed of as biohazardous material in accordance with
local or federal laws.
43
CX50 User Manual
4535 616 38521
2
Safety

• The backlight lamps in the system displays contain mercury and must be
recycled or disposed of according to local, state, or federal laws.
• Select the correct application when starting an exam, and remain in that
application throughout the exam. Some applications are for parts of the
body that require lower limits for acoustic output. One example is an
ophthalmic application activated by selecting an orbital transcranial Doppler
preset; when performing an ophthalmic exam, use only an ophthalmic preset.
• When used off the cart, the AC adapter and the system should not be
placed on the floor or on a patient’s bed. You can place it on a table or
chair.
FDA Medical Alert on Latex
March 29, 1991, Allergic Reactions to Latex-Containing Medical Devices
Because of reports of severe allergic reactions to medical devices containing
latex (natural rubber), the FDA is advising health care professionals to identify
their latex sensitive patients and be prepared to treat allergic reactions promptly.
Patient reactions to latex have ranged from contact urticaria to systemic
anaphylaxis. Latex is a component of many medical devices, including surgical
and examination gloves, catheters, intubation tubes, anesthesia masks, and dental
dams.
Reports to the FDA of allergic reactions to latex-containing medical devices have
increased lately. One brand of latex cuffed enema tips was recently recalled after
several patients died as a result of anaphylactoid reactions during barium enema
procedures. More reports of latex sensitivity have also been found in the medical
literature. Repeated exposure to latex both in medical devices and in other
consumer products may be part of the reason that the prevalence of latex
sensitivity appears to be increasing. For example, it has been reported that 6%
to 7% of surgical personnel and 18% to 40% of spina bifida patients are latex
sensitive.
Proteins in the latex itself appear to be the primary source of the allergic
reactions. Although it is not now known how much protein is likely to cause
CX50 User Manual
44
4535 616 38521
Safety
2

severe reactions, the FDA is working with manufacturers of latex-containing
medical devices to make protein levels in their products as low as possible.
FDA’s recommendations to health professionals in regard to this problem are
as follows:
• When taking general histories of patients, include questions about latex
sensitivity. For surgical and radiology patients, spina bifida patients and health
care workers, this recommendation is especially important. Questions about
itching, rash or wheezing after wearing latex gloves or inflating a toy balloon
may be useful. Patients with positive histories should have their charts
flagged.
• If latex sensitivity is suspected, consider using devices made with alternative
materials, such as plastic. For example, a health professional could wear a
non-latex glove over the latex glove if the patient is sensitive. If both the
health professional and the patient are sensitive, a latex middle glove could
be used. (Latex gloves labeled “Hypoallergenic” may not always prevent
adverse reactions.)
• Whenever latex-containing medical devices are used, especially when the
latex comes in contact with mucous membranes, be alert to the possibility
of an allergic reaction.
• If an allergic reaction does occur and latex is suspected, advise the patient
of a possible latex sensitivity and consider an immunologic evaluation.
• Advise the patient to tell health professionals and emergency personnel
about any known latex sensitivity before undergoing medical procedures.
Consider advising patients with severe latex sensitivity to wear a medical
identification bracelet.
The FDA is asking health professionals to report incidents of adverse reactions
to latex or other materials used in medical devices. (See the October 1990 FDA
Drug Bulletin.) To report an incident, contact the FDA Problem Reporting
Program, MedWatch, at 1-800-332-1088, or on the Internet:
www.fda.gov/Safety/MedWatch/
For a single copy of a reference list on latex sensitivity, write to: LATEX, FDA,
HFZ-220, Rockville, MD 20857.
45
CX50 User Manual
4535 616 38521
2
Safety

NOTE
The ultrasound system and transducers described in this document do not
contain natural rubber latex that contacts humans. Natural rubber latex is not
used on any Philips ultrasound transducer. It also is not used on Philips ECG
cables for the products described in this document.
ALARA Education Program
The guiding principle for the use of diagnostic ultrasound is defined by the «as
low as reasonably achievable» (ALARA) principle. The decision as to what is
reasonable has been left to the judgment and insight of qualified personnel. No
set of rules can be formulated that would be sufficiently complete to dictate the
correct response to every circumstance. By keeping ultrasound exposure as low
as possible, while obtaining diagnostic images, users can minimize ultrasonic
bioeffects.
Since the threshold for diagnostic ultrasound bioeffects is undetermined, it is
the sonographer’s responsibility to control total energy transmitted into the
patient. The sonographer must reconcile exposure time with diagnostic image
quality. To ensure diagnostic image quality and limit exposure time, an ultrasound
system provides controls that can be manipulated during the exam to optimize
the results of the exam.
The ability of the user to abide by the ALARA principle is important. Advances
in diagnostic ultrasound, not only in the technology but in the applications of
that technology, have resulted in the need for more and better information to
guide the user. The output display indices are designed to provide that important
information.
There are a number of variables which affect the way in which the output display
indices can be used to implement the ALARA principle. These variables include
index values, body size, location of the bone relative to the focal point, attenuation
in the body, and ultrasound exposure time. Exposure time is an especially useful
variable, because it is controlled by the user. The ability to limit the index values
over time supports the ALARA principle.
CX50 User Manual
46
4535 616 38521
Safety
2

Applying ALARA
The system imaging mode used depends upon the information needed. 2D and
M-mode imaging provide anatomical information, while Doppler, Color Power
Angio (CPA), and Color imaging provide information about blood flow. A scanned
mode, like 2D or Color, disperses or scatters the ultrasonic energy over an area,
while an unscanned mode, like M-mode or Doppler, concentrates ultrasonic
energy. Understanding the nature of the imaging mode being used allows the
sonographer to apply the ALARA principle with informed judgment. Additionally,
the transducer frequency, system setup values, scanning techniques, and operator
experience allow the sonographer to meet the definition of the ALARA principle.
The decision as to the amount of acoustic output is, in the final analysis, up to
the system operator. This decision must be based on the following factors: type
of patient, type of exam, patient history, ease or difficulty of obtaining
diagnostically useful information, and the potential localized heating of the patient
due to transducer surface temperatures. Prudent use of the system occurs when
patient exposure is limited to the lowest index reading for the shortest amount
of time necessary to achieve acceptable diagnostic results.
Although a high index reading does not mean that a bioeffect is actually occurring,
a high index reading should be taken seriously. Every effort should be made to
reduce the possible effects of a high index reading. Limiting exposure time is an
effective way to accomplish this goal.
There are several system controls that the operator can use to adjust the image
quality and limit the acoustic intensity. These controls are related to the
techniques that an operator might use to implement ALARA. These controls
can be divided into three categories: direct, indirect, and receiver controls.
Acoustic Output Limits
This ultrasound system maintains acoustic output below the appropriate limits
for each application, as listed here. The significant difference in magnitude
emphasizes the need to select the correct application and remain in that
application, so the correct application limits are in use for the appropriate
application.
47
CX50 User Manual
4535 616 38521
2
Safety

Limits for Non-Ophthalmic Applications
• I
spta.3
≤ 720 mW/cm
2
• MI ≤ 1.9
• TI ≤ 6.0
Limits for Ophthalmic Applications
• I
spta.3
≤ 50 mW/cm
2
• MI ≤ 0.23
• TI ≤ 1.0
Direct Controls
Application selection and the output-power control directly affect acoustic
intensity. There are different ranges of allowable intensity or output based on
your selection. Selecting the correct range of acoustic intensity for the application
is one of the first things that occurs in any exam. For example, peripheral vascular
intensity levels are not recommended for fetal exams. Some systems automatically
select the proper range for a particular application, while others require manual
selection. Ultimately, the user has the responsibility for proper clinical use. The
ultrasound system provides both automatic (default) settings and manual
(user-selectable) settings.
Output power has direct impact on acoustic intensity. Once the application has
been established, the power control can be used to increase or decrease the
intensity output. The power control allows you to select intensity levels less
than the established maximum. Prudent use dictates that you select the lowest
output intensity that is consistent with good image quality.
Indirect Controls
The indirect controls are those that have an indirect effect on acoustic intensity.
These controls affect imaging mode, pulse repetition frequency, focus depth,
pulse length, and transducer selection.
The choice of imaging mode determines the nature of the ultrasound beam. 2D
is a scanning mode; Doppler is a stationary or unscanned mode. A stationary
ultrasound beam concentrates energy in a single location. A moving or scanned
CX50 User Manual
48
4535 616 38521
Safety
2

ultrasound beam disperses the energy over an area and the beam is concentrated
on the same area for a fraction of the time as that of an unscanned mode.
Pulse repetition frequency or rate refers to the number of ultrasound bursts of
energy over a specific period of time. The higher the pulse repetition frequency,
the more pulses of energy in a period of time. Several controls affect pulse
repetition frequency: focal depth, display depth, sample volume depth, flow
optimization, scale, number of focal zones, and sector-width controls.
Focus of the ultrasound beam affects the image resolution. To maintain or
increase resolution at a different focus requires a variation in output over the
focal zone. This variation of output is a function of system optimization. Different
exams require different focal depths. Setting the focus at the proper depth
improves the resolution of the structure of interest.
Pulse length is the time during which the ultrasonic burst is turned on. The longer
the pulse, the greater the time-average intensity value. The greater the
time-average intensity, the greater the likelihood of temperature increase and
cavitation. Pulse length, burst length, or pulse duration is the output pulse
duration in PW Doppler. Increasing the Doppler sample-volume size increases
the pulse length.
Transducer selection indirectly affects intensity. Tissue attenuation changes with
frequency. The higher the transducer operating frequency, the greater the
attenuation of the ultrasonic energy. A higher transducer operating frequency
requires more output intensity to scan at a deeper depth. To scan deeper at the
same output intensity, a lower transducer frequency is required. Using more
gain and output beyond a point, without corresponding increases in image quality,
can mean that a lower frequency transducer is needed.
Receiver Controls
Receiver controls are used by the operator to improve image quality. These
controls have no effect on output. Receiver controls only affect how the
ultrasound echo is received. These controls include gain, TGC, dynamic range,
and image processing. The important thing to remember, relative to output, is
that receiver controls should be optimized before output is increased. For
example, before increasing output, optimize gain to improve image quality.
49
CX50 User Manual
4535 616 38521
2
Safety

An Example of Applying the ALARA Principle
An ultrasound scan of a patient’s liver begins with selecting the appropriate
transducer frequency. After selecting the transducer and the application, which
are based on patient anatomy, adjustments to output power should be made to
ensure that the lowest possible setting is used to acquire an image. After the
image is acquired, adjusting the focus of the transducer, and then increasing the
receiver gain to produce a uniform representation of the tissue follows. If an
adequate image can be obtained with the increase in gain, then a decrease in
output should be made. Only after making these adjustments should you increase
output to the next level.
Having acquired the 2D display of the liver, Color can be used to localize blood
flow. As with the 2D image display, gain and image processing controls must be
optimized before increasing output.
Having localized the blood flow, use the Doppler controls to position the sample
volume over the vessel. Before increasing output, adjust velocity range or scale
and Doppler gain to obtain an optimal Doppler trace. Only if maximum Doppler
gain does not create an acceptable image do you increase output.
In summary: Select the correct transducer frequency and application for the job;
start with a low output level; and optimize the image by using focus, receiver
gain, and other imaging controls. If the image is not diagnostically useful at this
point, then increase output.
Additional Considerations
Ensure that scanning time is kept to a minimum, and ensure that only medically
required scanning is performed. Never compromise quality by rushing through
an exam. A poor exam may require a follow-up, which ultimately increases
exposure time. Diagnostic ultrasound is an important tool in medicine, and like
any tool, it should be used efficiently and effectively.
Output Display
The system output display comprises two basic indices: a mechanical index and
a thermal index. The thermal index further consists of the following indices: soft
CX50 User Manual
50
4535 616 38521
Safety
2

CX50 U l t r a s o u n d S y s t e m
User Manual
4535 616 38521 Rev B
5HYLVLRQ
© 2012 Koninklijke Philips Electronics N.V. All rights reserved. Published in USA.

Manufactured by Philips Ultrasound
22100 Bothell-Everett Highway
Bothell, WA 98021-8431
USA
Telephone: +1 425-487-7000 or 800-426-2670
Fax: +1 425-485-6080
www.healthcare.philips.com/ultrasound
This Medical Device meets the provisions of the transposition of the Medical
Device Directive 93/42/EEC within the country of origin of the Notified Body
concerned with the device.
European Union Representative
Philips Medical Systems Nederland B.V.
Quality & Regulatory Affairs
Veenpluis 4-6
5684PC Best
The Netherlands
WARNING
United States federal law restricts this device to sale by or on the order of a
physician.
This document and the information contained in it is proprietary and confidential information of Philips Healthcare
(«Philips») and may not be reproduced, copied in whole or in part, adapted, modified, disclosed to others, or disseminated
without the prior written permission of the Philips Legal Department. This document is intended to be used by customers
and is licensed to them as part of their Philips equipment purchase. Use of this document by unauthorized persons is
strictly prohibited.
Philips provides this document without warranty of any kind, implied or expressed, including, but not limited to, the
implied warranties of merchantability and fitness for a particular purpose.
Philips has taken care to ensure the accuracy of this document. However, Philips assumes no liability for errors or
omissions and reserves the right to make changes without further notice to any products herein to improve reliability,
function, or design. Philips may make improvements or changes in the products or programs described in this document
at any time.
Unauthorized copying of this document, in addition to infringing copyright, might reduce the ability of Philips to provide
accurate and current information to users.
This product may contain remanufactured parts equivalent to new in performance, or parts that have had incidental use.
Philips Ultrasound products may be manufactured under or operate in accordance with one or more of the following
United States patents and corresponding patents in other countries: U.S. Patent Numbers 5,798,461; 6,450,958; 6,471,649;
6,527,721; 6,540,685; 6,572,547; 6,679,849. Other patent applications are pending in various countries.
«Chroma,» «Color Power Angio,» «High Q,» «QLAB,» «SonoCT,» and «XRES» are trademarks of Koninklijke Philips
Electronics N.V.
Non-Philips product names may be trademarks of their respective owners.
CX50 User Manual
2
4535 616 38521

Contents
1 Read This First………………………………………………………………………………15
Intended Audience………………………………………………………………………………………………15
Intended Use………………………………………………………………………………………………………..15
Warnings………………………………………………………………………………………………………………16
Warning Symbols…………………………………………………………………………………………………17
User Information Components…………………………………………………………………………..17
Product Conventions…………………………………………………………………………………………..18
User Information Conventions……………………………………………………………………………19
Upgrades and Updates………………………………………………………………………………………..21
Customer Comments………………………………………………………………………………………….21
Supplies and Accessories…………………………………………………………………………………….21
Customer Service………………………………………………………………………………………………..22
Recycling, Reuse, and Disposal……………………………………………………………………………22
2 Safety……………………………………………………………………………………………25
Basic Safety…………………………………………………………………………………………………………..25
Electrical Safety……………………………………………………………………………………………………26
Defibrillators…………………………………………………………………………………………………..29
Fire Safety……………………………………………………………………………………………………….30
Mechanical Safety…………………………………………………………………………………………………30
Equipment Protection………………………………………………………………………………………….31
Product Compatibility………………………………………………………………………………………….32
Symbols………………………………………………………………………………………………………………..33
Biological Safety……………………………………………………………………………………………………42
FDA Medical Alert on Latex…………………………………………………………………………..44
ALARA Education Program……………………………………………………………………………46
Output Display……………………………………………………………………………………………….50
Control Effects………………………………………………………………………………………………..54
3
CX50 User Manual
4535 616 38521

Related Guidance Documents…………………………………………………………………………..57
Acoustic Output and Measurement………………………………………………………………….57
Acoustic Output Tables…………………………………………………………………………………….61
Acoustic Measurement Precision and Uncertainty…………………………………………..61
Operator Safety………………………………………………………………………………………………………63
Repetitive Strain Injury …………………………………………………………………………………….63
Philips Transducers…………………………………………………………………………………………….63
Glutaraldehyde Exposure…………………………………………………………………………………..64
Infection Control……………………………………………………………………………………………….64
Electromagnetic Compatibility ………………………………………………………………………………66
Radio-Frequency Emissions……………………………………………………………………………….67
ECG Signal………………………………………………………………………………………………………….68
Electrostatic Discharge Precautions………………………………………………………………….69
Electromagnetic Emissions………………………………………………………………………………..70
Approved Cables for Electromagnetic Compliance………………………………………….70
Approved Transducers for Electromagnetic Compliance…………………………………71
Approved Accessories for Electromagnetic Compliance…………………………………72
Electromagnetic Immunity…………………………………………………………………………………72
Electromagnetic Interference…………………………………………………………………………….75
Recommended Separation Distance…………………………………………………………………78
Avoiding Electromagnetic Interference……………………………………………………………..80
Use Restrictions Due to Interference………………………………………………………………81
3 System Overview…………………………………………………………………………….83
System Capabilities…………………………………………………………………………………………………83
Measurements……………………………………………………………………………………………………83
Transducer Types……………………………………………………………………………………………….84
Image Acquisition and Review…………………………………………………………………………..84
Patient Data Protection…………………………………………………………………………………….85
System Options………………………………………………………………………………………………………85
Imaging Options…………………………………………………………………………………………………85
CX50 User Manual
4
4535 616 38521
Contents

Connectivity Options………………………………………………………………………………………..86
Clinical/Analysis Options…………………………………………………………………………………..86
Calculations………………………………………………………………………………………………………..87
QLAB Advanced Quantification Software Options………………………………………….87
Stress Echocardiography……………………………………………………………………………………88
Data Security……………………………………………………………………………………………………..88
System Components………………………………………………………………………………………………88
Video Monitor……………………………………………………………………………………………………90
Control Panel…………………………………………………………………………………………………….90
On/Off (Power) Control……………………………………………………………………………………91
Data Storage ……………………………………………………………………………………………………..92
Peripherals………………………………………………………………………………………………………….93
Transducer Receptacles and Cable Management……………………………………………..93
Physio (ECG) Receptacles…………………………………………………………………………………95
USB Hub…………………………………………………………………………………………………………….96
Wheel Controls…………………………………………………………………………………………………97
4 Preparing the System………………………………………………………………………99
Connecting Devices………………………………………………………………………………………………..99
External Printers………………………………………………………………………………………………100
Connecting an External Printer………………………………………………………………………102
Configuring Local Printers………………………………………………………………………………103
Connecting the Optional Foot Switch……………………………………………………………104
Configuring the Foot Switch……………………………………………………………………………104
Connecting an External Color Monitor ………………………………………………………..104
Attaching the System……………………………………………………………………………………………105
Removing the System……………………………………………………………………………………………106
System Configuration……………………………………………………………………………………………106
Standard Network Support…………………………………………………………………………….107
DICOM Networking Option…………………………………………………………………………..107
Configuration Information………………………………………………………………………………107
5
CX50 User Manual
4535 616 38521
Contents

Entering System Network Settings…………………………………………………………………109
Changing the PC Name…………………………………………………………………………………..111
Wireless Networking………………………………………………………………………………………112
Configuring Wireless Network Properties…………………………………………………….112
Enabling a Wireless Network Connection……………………………………………………..115
Removing a Wireless Network……………………………………………………………………….118
Troubleshooting Wireless Network Connections…………………………………………119
Remote Access………………………………………………………………………………………………..119
Enabling a Remote Access Session………………………………………………………………….120
Repairing Network Connections…………………………………………………………………….120
Moving the System………………………………………………………………………………………………..121
Preparing and Moving………………………………………………………………………………………122
Setting Up After Moving………………………………………………………………………………….123
5 Using the System…………………………………………………………………………..125
Turning the System On and Off……………………………………………………………………………125
Setting the System Time and Date……………………………………………………………………….126
System Cart…………………………………………………………………………………………………………..127
Installing the AC Adapter………………………………………………………………………………..127
Attaching the System……………………………………………………………………………………….130
Removing the System………………………………………………………………………………………130
Adjusting Cart Height……………………………………………………………………………………..130
Using the Wheel Controls………………………………………………………………………………132
Monitor Settings……………………………………………………………………………………………………133
Changing the Monitor Tint……………………………………………………………………………..134
Changing the Monitor Brightness……………………………………………………………………134
System Controls……………………………………………………………………………………………………135
Control Panel…………………………………………………………………………………………………..135
Control Status………………………………………………………………………………………………….136
Changing Control Panel Brightness…………………………………………………………………137
Enabling Automatic Brightness Control …………………………………………………………138
CX50 User Manual
6
4535 616 38521
Contents

Quick Key Controls…………………………………………………………………………………………138
Using Quick Key Controls………………………………………………………………………………139
System Keyboard……………………………………………………………………………………………..139
Typing Special Characters………………………………………………………………………………..140
Typing Accented Characters……………………………………………………………………………140
Status Icons………………………………………………………………………………………………………141
Power Management………………………………………………………………………………………………143
Battery and AC Indicators………………………………………………………………………………144
Changing Power Management Settings……………………………………………………………145
AC Adapter Operation ……………………………………………………………………………………….146
AC Adapter Indicator………………………………………………………………………………………147
Using the AC Adapter……………………………………………………………………………………..147
Battery Operation………………………………………………………………………………………………..148
Installing the Battery ………………………………………………………………………………………149
System Security…………………………………………………………………………………………………….151
Logging On to the System……………………………………………………………………………….151
Logging Off of the System……………………………………………………………………………….151
System and Data Security………………………………………………………………………………..152
Emergency Studies………………………………………………………………………………………………..153
Temporary ID…………………………………………………………………………………………………..153
Starting Emergency Studies……………………………………………………………………………..154
Imaging Display……………………………………………………………………………………………………..155
Image Size Settings………………………………………………………………………………………………..157
Transducer Receptacles and Cable Management…………………………………………………157
Connecting Transducers………………………………………………………………………………….159
Selecting a Transducer……………………………………………………………………………………..161
Selecting a Preset…………………………………………………………………………………………….162
Using Presets……………………………………………………………………………………………………162
Physio Feature……………………………………………………………………………………………………….163
DVD, CD, and USB Devices…………………………………………………………………………………164
7
CX50 User Manual
4535 616 38521
Contents

Media Compatibility…………………………………………………………………………………………164
Loading and Ejecting a Disc……………………………………………………………………………..165
USB Devices…………………………………………………………………………………………………….165
Erasing a DVD or USB Device………………………………………………………………………..167
6 Customizing the System………………………………………………………………..169
Presets…………………………………………………………………………………………………………………..169
Clinical Options and Predefined Presets…………………………………………………………169
Custom Presets……………………………………………………………………………………………….170
Creating Custom Presets………………………………………………………………………………..170
Modifying Custom Presets………………………………………………………………………………171
Deleting Custom Presets………………………………………………………………………………..172
Setting Up Autoselect Presets…………………………………………………………………………172
Presets Menu……………………………………………………………………………………………………173
Using the Presets Menu…………………………………………………………………………………..173
Modifying the Presets Menu…………………………………………………………………………….174
Copying Custom Presets…………………………………………………………………………………175
System Setups……………………………………………………………………………………………………….175
Changing Setups……………………………………………………………………………………………….176
Options…………………………………………………………………………………………………………………176
Installing Temporary Options………………………………………………………………………….176
7 Performing a Study………………………………………………………………………..179
New Patient Studies……………………………………………………………………………………………..179
Entering Patient Data Manually (Without Worklist)………………………………………180
Using Modality Worklist………………………………………………………………………………….181
Selecting a Transducer………………………………………………………………………………………….182
Imaging Modes………………………………………………………………………………………………………182
Using 2D Mode………………………………………………………………………………………………..183
Annotation…………………………………………………………………………………………………………….184
Placing a System-Defined Label on the Display………………………………………………184
Typing a Label on the Display………………………………………………………………………….185
CX50 User Manual
8
4535 616 38521
Contents

Placing a Body Marker on the Display…………………………………………………………….185
Printing………………………………………………………………………………………………………………….186
Review…………………………………………………………………………………………………………………..186
Starting Review………………………………………………………………………………………………..187
Navigating Thumbnails and Images………………………………………………………………….187
Acquiring Images and Loops ……………………………………………………………………………….188
Measurement and Analysis……………………………………………………………………………………189
Performing a 2D Distance Measurement………………………………………………………..190
Obtaining a Typical Labeled Measurement……………………………………………………..190
Obtaining a Calculation Result………………………………………………………………………..191
Ending a Study……………………………………………………………………………………………………….191
8 Transducers……………………………………………………………………………………193
Selecting a Transducer………………………………………………………………………………………….194
Selecting a Preset………………………………………………………………………………………………….194
Clinical Options and Transducers………………………………………………………………………..195
Indications for Use and Supporting Transducers…………………………………………………196
Transducer Maintenance……………………………………………………………………………………….198
Acoustic Artifacts…………………………………………………………………………………………………199
Acoustic Artifacts in 3D Imaging…………………………………………………………………….202
Transducer Covers……………………………………………………………………………………………….204
Transducer Storage……………………………………………………………………………………………….205
Storage for Transport …………………………………………………………………………………….205
Daily and Long-Term Storage………………………………………………………………………….205
9 Endocavity Transducers………………………………………………………………….207
Operators of Endocavity Transducers…………………………………………………………………207
Patient Safety During Endocavity Studies…………………………………………………………….207
Preparing Transducers for Endocavity Use………………………………………………………….208
C9-3v Description………………………………………………………………………………………………..209
C10-3v Description………………………………………………………………………………………………210
Patient-Contact Parts……………………………………………………………………………………………211
9
CX50 User Manual
4535 616 38521
Contents

Biopsy with Endocavity Transducers…………………………………………………………………….212
10 Transesophageal Transducers…………………………………………………………213
Operators of TEE Transducers…………………………………………………………………………….213
Patient Safety During TEE Studies………………………………………………………………………..213
Patient-Contact Parts………………………………………………………………………………………218
Preventing TEE Transducer Problems………………………………………………………………….219
Electrical Safety and TEE Transducers………………………………………………………………….221
Leakage Current and TEE Transducers…………………………………………………………..221
Reducing Risks of Using TEE Transducers………………………………………………………221
TEE Deflection Control Basics ……………………………………………………………………………222
Connecting a TEE Transducer………………………………………………………………………………224
X7-2t TEE Transducer Description……………………………………………………………………..224
TEE Transducer Components………………………………………………………………………………225
TEE Deflection Controls…………………………………………………………………………………227
Manipulating the TEE Tip…………………………………………………………………………………229
Rotating the TEE Image Plane ………………………………………………………………………..231
Checking the TEE Transducer…………………………………………………………………………232
Special Considerations for TEE Studies……………………………………………………………….233
Patient Selection for TEE Transducer Use……………………………………………………..233
Preparing Patients for TEE Studies………………………………………………………………….234
TEE Study Guidelines………………………………………………………………………………………235
Tip Fold-Over……………………………………………………………………………………………………….236
Recognizing Tip Fold-Over……………………………………………………………………………..236
Correcting Tip Fold-Over……………………………………………………………………………….236
Preventing Tip Fold-Over ……………………………………………………………………………….236
TEE Temperature Sensing…………………………………………………………………………………….237
Ensuring Safe TEE Temperatures…………………………………………………………………….238
Manual Auto-Cool Feature……………………………………………………………………………..239
Patient Temperature………………………………………………………………………………………..240
Entering Patient Temperature………………………………………………………………………….240
CX50 User Manual
10
4535 616 38521
Contents

Temperature Display………………………………………………………………………………………..241
Customizing the Temperature Display……………………………………………………………241
Resuming Imaging After Auto-Cool………………………………………………………………..242
Patient Care After a TEE Study……………………………………………………………………………243
TEE Accessories and Supplies………………………………………………………………………………243
Bite Guards………………………………………………………………………………………………………243
TEE Transducer Covers…………………………………………………………………………………..244
Tip Protectors………………………………………………………………………………………………….244
Disposable Drapes…………………………………………………………………………………………..244
TEE Leakage Current Test……………………………………………………………………………………244
TEE Test Background……………………………………………………………………………………….245
Testing TEE Transducer Leakage Current……………………………………………………….247
TEE Transducer References………………………………………………………………………………….248
11 Intraoperative Transducers……………………………………………………………249
Operators of Intraoperative Transducers……………………………………………………………249
Intended Uses for Intraoperative Transducers…………………………………………………….250
Patient Safety During Intraoperative Studies ………………………………………………………250
Patient-Contact Parts………………………………………………………………………………………251
Preventing Intraoperative Transducer Problems ………………………………………………..251
C9-3io Description……………………………………………………………………………………………….252
L10-4lap Description…………………………………………………………………………………………….254
L15-7io Description……………………………………………………………………………………………..256
Preparing Transducers for Intraoperative Use…………………………………………………….257
Disposable Drapes…………………………………………………………………………………………..258
Accessories for Intraoperative Transducers…………………………………………………..258
Electrical Safety and Intraoperative Transducers…………………………………………………258
Leakage Current Testing for Intraoperative Transducers……………………………………259
Testing Intraoperative Transducer Leakage Current (Source)……………………….264
Testing Intraoperative Transducer Leakage Current (Sink)……………………………264
12 ICE Catheter Transducer……………………………………………………………….267
11
CX50 User Manual
4535 616 38521
Contents

Connecting the ICE Catheter………………………………………………………………………………267
13 Biopsy Guides………………………………………………………………………………..269
Attaching and Removing a Biopsy Guide…………………………………………………………….269
Biopsy Guideline Display………………………………………………………………………………………270
Displaying the Biopsy Guideline………………………………………………………………………271
Moving the Needle Length Crosshair……………………………………………………………..272
Biopsy Guideline Quick Keys………………………………………………………………………….272
Biopsy Guide Alignment……………………………………………………………………………………….273
Preparation for Alignment Verification……………………………………………………………273
Verifying the Biopsy Guide Alignment…………………………………………………………….274
Performing a Biopsy Procedure……………………………………………………………………………276
Biopsy Guide Maintenance……………………………………………………………………………………277
Needle Visualization……………………………………………………………………………………………..278
Using Needle Visualization………………………………………………………………………………278
14 Transducer Care……………………………………………………………………………281
Transducer Care Safety………………………………………………………………………………………..281
Latex Product Alert…………………………………………………………………………………………282
Transmissible Spongiform Encephalopathy……………………………………………………..282
Acoustic Coupling Medium………………………………………………………………………………….283
Choosing a Disinfectant………………………………………………………………………………………..283
General Cleaning for All Transducers………………………………………………………………….284
Cleaning a Transducer……………………………………………………………………………………..285
Disinfecting Transducers Using a Wipe or Spray Method ………………………………….285
Cleaning and Disinfecting Cables and Connectors……………………………………………..288
Disinfection of Transducers by Immersion (High-Level Disinfection)………………..291
Disinfecting Transducers by Immersion………………………………………………………….292
Disinfecting TEE Transducers by Immersion…………………………………………………..294
Disinfecting TEE Transducers in an Automated Disinfector………………………….297
Sterilizing Transducers………………………………………………………………………………………….300
Disinfectants Compatibility…………………………………………………………………………………..302
CX50 User Manual
12
4535 616 38521
Contents

Disinfectant Types……………………………………………………………………………………………303
Factors Affecting Disinfectant Efficiency…………………………………………………………303
Disinfectants and Cleaning Solutions Compatibility Table……………………………..304
Gels Compatibility………………………………………………………………………………………………..311
15 System Maintenance……………………………………………………………………..313
Cleaning and Maintaining the System…………………………………………………………………..313
Cleaning the System and ECG Equipment……………………………………………………..313
Disinfectants for System Surfaces……………………………………………………………………315
Disinfecting System Surfaces……………………………………………………………………………315
Cleaning the Trackball……………………………………………………………………………………..316
Cleaning the Battery………………………………………………………………………………………..317
Cleaning the Adapter………………………………………………………………………………………317
Transducer Maintenance……………………………………………………………………………………….318
Printer Maintenance……………………………………………………………………………………………..319
Troubleshooting…………………………………………………………………………………………………….319
Error Messages……………………………………………………………………………………………………..320
For Assistance……………………………………………………………………………………………………….321
16 Specifications…………………………………………………………………………………323
Safety and Regulatory Requirements…………………………………………………………………..327
Index……………………………………………………………………………………………..329
13
CX50 User Manual
4535 616 38521
Contents

CX50 User Manual
14
4535 616 38521
Contents

1 Read This First
This manual is intended to assist you with the safe and effective operation of
your Philips product. Before attempting to operate the product, read this
manual and strictly observe all warnings and cautions. Pay special attention to
the information in the
«Safety» section.
The user information for your Philips product describes the most extensive
configuration of the product, with the maximum number of options and
accessories. Some functions described may be unavailable on your product’s
configuration.
Intended Audience
Before you use your user information, you need to be familiar with ultrasound
techniques. Sonography training and clinical procedures are not included here.
This document is intended for sonographers, physicians, and biomedical
engineers who operate and maintain your Philips product.
Intended Use
This product is intended to be installed, used, and operated only in accordance
with the safety procedures and operating instructions given in the product
user information, and only for the purposes for which it was designed. For
indications for use, see
«Indications for Use and Supporting Transducers» on
page 196
. However, nothing stated in the user information reduces your
responsibility for sound clinical judgment and best clinical procedure.
Installation, use, and operation of this product is subject to the law in the
jurisdictions in which the product is used. Install, use, and operate the product
only in such ways that do not conflict with applicable laws or regulations, which
have the force of law.
15
CX50 User Manual
4535 616 38521

Use of the product for purposes other than those intended and expressly stated
by Philips, as well as incorrect use or operation, may relieve Philips or its agents
from all or some responsibility for resultant noncompliance, damage, or injury.
WARNING
System users are responsible for image quality and diagnosis.
Warnings
Before using the system, read these warnings and the «Safety» section.
WARNINGS
• Do not remove the protective covers on the system; hazardous voltages
are present inside. Cabinet panels must be in place while the system is in
use. All internal adjustments and replacements must be made by a qualified
Philips Ultrasound field service engineer.
• To avoid electrical shock, use only supplied power cords and connect only
to properly grounded wall (wall/mains) outlets.
• Do not operate the system in the presence of flammable anesthetics or
other flammable gases or liquids. Explosion can result.
• Medical equipment must be installed and put into service according to the
special electromagnetic compatibility (EMC) guidelines provided in the
«Safety» section.
• The use of portable and mobile radio-frequency (RF) communications
equipment can affect the operation of medical equipment.
CX50 User Manual
16
4535 616 38521
Read This First
1

Warning Symbols
The system may use the following warning symbols. For additional symbols used
on the system, see the
«Safety» section.
DescriptionSymbol
Identifies a safety note.
Dangerous voltages: Appears adjacent to high-voltage terminals,
indicating the presence of voltages greater than 1,000 Vac (600 Vac in
the United States).
Identifies ESD (electrostatic-discharge) sensitivity of a connector that
is not tested as specified in IEC 60601-1-2. Do not touch exposed
connector pins. Touching exposed pins can cause electrostatic
discharge, which can damage the product.
Indicates that the user should see the instructions for use for safety
information.
User Information Components
The user information provided with your product includes the following
components:
• Compact Disc (CD): Includes all of the user information, except the
Operating Notes. The instructions for using the CD are included with the
CD.
• Operating Notes: Contains information that clarifies certain product
responses that might be misunderstood or cause user difficulty.
• User Manual: Provided with the product and included on the CD. The
User Manual introduces you to features and concepts, helps you set up your
system, and includes important safety information. This manual also includes
17
CX50 User Manual
4535 616 38521
1
Read This First

procedures for basic operation. For detailed operating instructions, see the
Help.
• CX50 Integrated Ultrasound User Manual: This manual introduces you
to Integrated Ultrasound, helps you set it up, and provides basic operating
instructions. For more information on the use and operation of your Allura
XPer FD system with Integrated Ultrasound, see the Allura XPer FD
instructions for use.
• Help: Available on the system in some languages and included on the CD,
the Help contains comprehensive instructions for using the system. The
Help also provides reference information and descriptions of all controls
and display elements. To display the Help, press Help on the system
keyboard.
• Acoustic Output Tables: Included on the CD, it contains information
about acoustic output and patient-applied part temperatures.
• Medical Ultrasound Safety: Included on the CD, it contains information
on bioeffects and biophysics, prudent use, and implementing ALARA (as
low as reasonably achievable).
• Shared Roles for System and Data Security: Included on the CD, it
contains guidelines to help you understand how the security of your Philips
product could be compromised and information on Philips’ efforts to help
you prevent security breaches.
• Media Compatibility: Included on the CD, it contains current information
on media that are compatible with your system.
Product Conventions
Your Philips product uses certain conventions throughout the interface to make
it easy for you to learn and use:
• Two unlabeled buttons, referred to as «trackball buttons,» are used with
the trackball. Those controls, located on either side of the trackball, operate
CX50 User Manual
18
4535 616 38521
Read This First
1

somewhat similarly to PC mouse buttons. Both trackball buttons function
identically.
• In the system setups, tabs along the top of the monitor display let you
choose additional sets of setup options.
• To type text into a text field, click in the field and use the keyboard.
•
To display a list, click the down arrow
. To scroll through a list, click the
arrows at either end of the scroll bar or drag the scroll box up or down.
• Controls on the control panel include buttons, knobs, slide controls, and
a trackball. Press a button to activate or deactivate its function. Turn a knob
to change the selected setting. Move a slide control to change its setting.
Roll the trackball in the direction that you want to move an object. The
current trackball function is displayed in the trackball select menu at the
bottom of the display.
• Controls across the top of the control panel, called quick keys, function as
both buttons and knobs. To select one of the functions displayed above the
control, simply press the control. To select a setting for the function, also
displayed above the control, turn the control.
User Information Conventions
The user information for your product uses the following typographical
conventions to assist you in finding and understanding information:
• All procedures are numbered, and all subprocedures are lettered. You must
complete steps in the sequence they are presented to ensure success.
• Bulleted lists indicate general information about a particular function or
procedure. They do not imply a sequential procedure.
• Control names and menu items or titles are spelled as they are on the
system, and they appear in bold text. The only exceptions are the trackball
and the buttons adjacent to it, which are unlabeled.
• Symbols appear as they appear on the system.
• The pointer is the cursor used to select elements on the display. Use the
Pointer control to display the pointer.
• Point means to position the tip of the pointer or cursor on an item on the
display.
19
CX50 User Manual
4535 616 38521
1
Read This First

• Click means to move the pointer to an object and press the left trackball
button.
• Select means to click a check box to put a check mark in it. Deselect means
clicking the check box to remove the check mark.
• Double-click means to quickly click twice to select an object or text.
• Right-click means to point at an item and then press and immediately release
the right trackball button.
• Hover means to pause the pointer over an item on the display.
• Drag means to place the pointer over an object and then press and hold
the left trackball button while moving the trackball. Use this method to
move an object on the display.
• Highlight means to change the color of a display selection (such as an item
in a list) or overlay it with a colored bar, usually by clicking.
• The left side of the system is to your left as you stand in front of the system,
facing the system. The front of the system is nearest to you as you operate
it.
• Transducers and pencil probes both are referred to as transducers, unless
the distinction is important to the meaning of the text.
Information that is essential for the safe and effective use of your product appears
throughout your user information as follows:
WARNING
Warnings highlight information vital to the safety of you, the operator, and the
patient.
CAUTION
Cautions highlight ways that you could damage the product and consequently
void your warranty or service contract or ways that you could lose patient or
system data.
NOTE
Notes bring your attention to important information that will help you operate
the product more effectively.
CX50 User Manual
20
4535 616 38521
Read This First
1

Upgrades and Updates
Philips is committed to innovation and continued improvement. Upgrades may
be announced that consist of hardware or software improvements. Updated
user information will accompany those upgrades.
Customer Comments
If you have questions about the user information, or you discover an error in
the user information, in the USA, please call Philips at 800-722-9377; outside
the USA, please call your local customer service representative.
Supplies and Accessories
To order ECG trunk cables, lead sets, and electrodes; transducer covers; bite
guards; biopsy guides; and other supplies and accessories, contact CIVCO Medical
Solutions:
CIVCO Medical Solutions
102 First Street South, Kalona, IA 52247-9589
Telephone: 800-445-6741 (USA and Canada), +1 319-248-6757 (International)
Fax: 877-329-2482 (USA and Canada), +1 319-248-6660 (International)
E-mail: info@civco.com
Internet: www.civco.com
To order the items listed in the following table, see the referenced section and
then contact your Philips representative.
21
CX50 User Manual
4535 616 38521
1
Read This First

System Accessories
Additional InformationItem
Contact your Philips representative.Battery
See
«Approved Cables for Electromagnetic
Compliance» on page 70
Cables
Contact your Philips representative.Foot switch
See «External Printers» on page 100Printers
See
«Media Compatibility» on page 164Removable media
See
«Clinical Options and Transducers» on
page 195
Transducers
Customer Service
Customer service representatives are available worldwide to answer questions
and to provide maintenance and service. Please contact your local Philips
representative for assistance. You can also contact the following office for referral
to a customer service representative, or visit the Philips Healthcare «Contact
Us» website:
www.healthcare.philips.com/main/about/officelocator/index.wpd
Philips Ultrasound Headquarters
22100 Bothell-Everett Highway, Bothell, WA 98021-8431, USA
800-722-9377
Recycling, Reuse, and Disposal
Philips is concerned with helping protect the natural environment and helping
ensure continued safe and effective use of this system through proper support,
maintenance, and training. Philips designs and manufactures equipment in
compliance with relevant guidelines for environmental protection. As long as
CX50 User Manual
22
4535 616 38521
Read This First
1

the equipment is properly operated and maintained, it presents no risk to the
environment. However, the equipment may contain materials that could be
harmful to the environment if disposed of incorrectly. Use of such materials is
essential for the implementation of certain functions and for meeting certain
statutory and other requirements.
The European Union Directive on Waste Electrical and Electronic Equipment
(WEEE) requires producers of electrical and electronic equipment to provide
reuse and treatment information for each product. This information is provided
in a Philips Healthcare Recycling Passport. Such recycling passports for Philips
Ultrasound systems are available on this website:
www.healthcare.philips.com/main/about/sustainability/recycling/ultrasound.wpd
Recycling, reuse, and disposal information in this document is directed mainly at
the entity with legal authority over the equipment. Operators are usually
uninvolved in disposal, except in the case of certain batteries (see
«Battery
Operation» on page 148
).
Passing Your System to Another User
If you pass this system to another user who will use the system for its intended
purpose, then pass it on in its complete state. Particularly, ensure that all the
product-support documentation, including all instructions for use, are passed on
to the new user. Make the new user aware of the support services that Philips
Healthcare provides for installing, commissioning, and maintaining the system,
and for comprehensive operator training. Existing users must remember that
passing on medical electrical equipment to new users may present serious
technical, medical, privacy, and legal risks. The original user may remain liable,
even if the equipment is given away.
Philips strongly advises you to seek advice from your local Philips representative
before agreeing to pass on any equipment.
After you pass the system to a new user, you might still receive important
safety-related information, such as bulletins and field change orders. In many
jurisdictions the original owner has a clear duty to communicate such
safety-related information to new users. If you are unable or unprepared to do
23
CX50 User Manual
4535 616 38521
1
Read This First

this, inform Philips Healthcare about the new user, so that Philips Healthcare
can provide the new user with safety-related information.
Final Disposal of Your System
Final disposal is when you dispose of the system in such a way that it can no
longer be used for its intended purposes.
WARNING
Do not dispose of this system (or any parts of it) with industrial or domestic
waste. The system may contain materials such as lead, tungsten, or oil, or other
hazardous substances that can cause serious environmental pollution. The system
also contains privacy-sensitive information, which should be properly removed
(scrubbed). Philips advises you to contact your Philips service organization before
disposing of this system.
Philips Healthcare gives support for the following:
• Recovery of useful parts
• Recycling of useful materials by competent disposal companies
• Safe and effective disposal of equipment
For advice and information, contact your Philips service organization, or see the
following website:
www.philips.com/about/sustainability/recycling/productrecyclingservices/index.page
Perchlorate Material
In this system, perchlorate material is present in lithium coin cells or batteries.
Special handling may apply to those items. For more information, see this website:
www.dtsc.ca.gov/hazardouswaste/perchlorate
CX50 User Manual
24
4535 616 38521
Read This First
1

2 Safety
Please read this information before using your ultrasound system. It applies to
the ultrasound system, transducers, recording devices, and any optional
equipment. This section covers general safety information only. Safety
information that applies only to a specific task is included in the procedure for
that task.
This device is intended for use by, or by the order of, and under the supervision
of a licensed physician qualified to direct the use of the device.
A WARNING describes precautions necessary to prevent injury or loss of
life.
A CAUTION describes precautions necessary to protect the equipment and
patient or system data.
Basic Safety
WARNINGS
• Do not use the system for any application until you have read, understood,
and know all the safety information, safety procedures, and emergency
procedures contained in this «Safety» section. Operating the system
without a proper awareness of safe use could lead to fatal or other serious
personal injury.
• Do not use this system for any application until you are sure that the
system’s periodic maintenance is current. If any part of the system is
known or suspected to be defective or incorrectly adjusted, do not use
the system until it is repaired. Operating the system with defective or
incorrectly adjusted components could expose you and the patient to
safety hazards.
• Do not use the system for any application until you are adequately and
properly trained on its safe and effective operation. If you are unsure of
your ability to operate the system safely and effectively, do not use it.
25
CX50 User Manual
4535 616 38521

Operation of the system without proper and adequate training could lead
to fatal or other serious personal injury.
• Do not operate the system with patients unless you have an adequate
understanding of its capabilities and functions. Using the system without
such understanding may compromise the system’s effectiveness and the
safety of the patient, you, and others.
• Never attempt to remove, modify, override, or frustrate any safety device
on the system. Interfering with safety devices could lead to fatal or other
serious personal injury.
• Use the system only for its intended purposes. Do not use the system with
any product that Philips does not recognize as compatible with the system.
Operation of the product for unintended purposes, or with incompatible
products, could lead to fatal or other serious injury.
Electrical Safety
This equipment has been verified by a recognized third-party testing agency as
a Class I device with Type BF and Type CF isolated patient-applied parts, and
Type B non-isolated patient-applied parts. (The safety standards met by this
system are included in the
«Specifications» section.) For maximum safety observe
these warnings and cautions:
WARNINGS
• Shock hazards may exist if this system (when mounted on its cart or plugged
directly into an AC power source), including all externally mounted
recording and monitoring devices, is not properly grounded. Protection
against electrical shock is provided by grounding the cart or the AC power
adapter with a three-wire cable and plug, which must be plugged into a
grounded outlet. The grounding wire must not be removed or defeated.
• To avoid the risk of electrical shock, never connect the system power cord
to a power strip or an extension cord. When using the power cord, always
connect it directly to a grounded wall outlet.
• Use only the AC adapter supplied with your system.
CX50 User Manual
26
4535 616 38521
Safety
2

• Use only Type CF transducers for invasive procedures. Type B transducers
are insufficiently electrically isolated for invasive use.
• Do not remove the protective covers on the system; hazardous voltages
are present inside. Cabinet panels must be in place while the system is in
use. All internal adjustments and replacements must be made by a qualified
Philips Ultrasound field service engineer.
• Do not operate this system in the presence of flammable gases or
anesthetics. Explosion can result. The system is not compliant in AP/APG
environments as defined by IEC 60601-1.
• To avoid risk of electrical shock hazards, always inspect the transducer
before use: Check the face, housing, and cable before use. Do not use if
the face is cracked, chipped, or torn; the housing is damaged; or the cable
is abraded.
• To avoid risk of electrical shock hazards, always turn off the system,
disconnect it from the wall outlet, and remove the battery (see
«Installing
the Battery» on page 149
) before cleaning the system.
• All patient-contact devices, such as transducers, pencil probes, and ECG
leads not specifically indicated as defibrillation-proof must be removed from
patient contact before application of a high-voltage defibrillation pulse. See
«Defibrillators» on page 29.
• During transesophageal echocardiographic (TEE) procedures, either remove
the TEE transducer from the patient or disconnect the TEE transducer from
the system immediately following image acquisition.
• Ultrasound equipment in normal operation, as with other medical electronic
diagnostic equipment, uses high-frequency electrical signals that can interfere
with pacemaker operation. Though the possibility of interference is slight,
be alert to this potential hazard and stop system operation immediately if
you note interference with a pacemaker.
• When using additional peripheral equipment powered from an electrical
source other than the ultrasound system, the combination is considered
to be a medical system. It is your responsibility to comply with
IEC 60601-1-1 and test the system to those requirements. If you have
questions, contact your Philips representative.
• Do not use nonmedical peripherals, such as report printers, within 1.5 m
(5 ft) of a patient, unless the nonmedical peripherals receive power from
27
CX50 User Manual
4535 616 38521
2
Safety

an isolated power outlet on the Philips ultrasound system, or from an
isolation transformer that meets medical safety standards, as defined by
standard IEC 60601-1-1.
• The system and patient-applied parts meet the standard IEC 60601-1.
Applied voltages exceeding the standard, although unlikely, may result in
electrical shock to the patient or operator.
• Connection of optional devices not supplied by Philips Ultrasound could
result in electrical shock. When such optional devices are connected to
your ultrasound system, ensure that the total system earth leakage current
does not exceed 500 µA, or in the United States, 300 µA.
• To avoid risk of electrical shock, do not use any transducer that has been
immersed beyond the specified cleaning or disinfection level.
• To avoid risks of electrical shock and fire hazards, inspect the system power
cord and plug regularly. Ensure that they are not damaged in any way.
• Do not drape the power cord over any of the cable hooks or the handle
on the system cart. Damage to the cord or power receptacle unit can occur
if the cart is raised.
• Operating the system with physio input signals that are below the specified
minimum levels may cause inaccurate results. See the
«Specifications» section.
• Electrosurgical units (ESUs) and other devices intentionally introduce radio
frequency electromagnetic fields or currents into patients. Because imaging
ultrasound frequencies are coincidentally in the radio frequency range,
ultrasound transducer circuits are susceptible to radio frequency
interference. While an ESU is in use, severe noise interferes with the
black-and-white image and completely obliterates the color image.
Concurrent failures in an ESU or other device and in the outer layer of the
TEE transducer shaft can cause electrosurgical currents to return along the
transducer conductors. This could burn the patient, and the ultrasound
system and the transducer could also be damaged. Be aware that a disposable
transducer cover provides no protective electrical insulation at ESU
frequencies.
• To avoid risk of a burn hazard, do not use transducers with high-frequency
surgical equipment. A burn hazard may result from a defect in the
high-frequency surgical neutral electrode connection.
CX50 User Manual
28
4535 616 38521
Safety
2

• Using cables, transducers, and accessories other than those specified for
use with the system may result in increased emissions from, or decreased
immunity of, the system.
CAUTIONS
• Although your system has been manufactured in compliance with existing
EMI/EMC requirements, use of this system in the presence of an
electromagnetic field can cause momentary degradation of the ultrasound
image. When interference is present or intermittent, use caution when
continuing to use the system. If interference occurs often, review the
environment in which the system is being used, to identify possible sources
of radiated emissions. These emissions could be from other electrical devices
used within the same room or an adjacent room. Communication devices
such as cellular phones and pagers can cause these emissions. The existence
of radio, TV, or microwave transmission equipment located nearby can
cause emissions. In cases where EMI is causing disturbances, it may be
necessary to relocate your system.
• For information on electromagnetic emissions and immunity as it applies
to the system, see
«Electromagnetic Compatibility» on page 66. Ensure that
the
operating environment of your system meets the conditions specified
in the referenced information. Operating the system in an environment that
does not meet those conditions may degrade system performance.
Defibrillators
Observe the following warnings when a defibrillation is required while using the
ultrasound system.
WARNINGS
• Before defibrillation, always remove all patient-applied parts from the patient.
• Before defibrillation, always disconnect invasive transducers from the system.
• A disposable transducer cover provides no protective electrical insulation
against defibrillation.
• A small hole in the outer layer of the transducer opens a conductive path
to grounded metal parts of the transducer. The secondary arcing that could
29
CX50 User Manual
4535 616 38521
2
Safety

occur during defibrillation could cause patient burns. The risk of burns is
reduced, but not eliminated, by using an ungrounded defibrillator.
Use defibrillators that do not have grounded patient circuits. To determine
whether a defibrillator patient circuit is grounded, see the defibrillator service
guide, or consult a biomedical engineer.
Fire Safety
WARNING
On electrical or chemical fires, use only extinguishers that are specifically labeled
for those purposes. Using water or other liquids on an electrical fire can lead to
fatal or other serious personal injury. Before attempting to fight a fire, if it is safe
to do so, attempt to isolate the product from electrical and other supplies, to
reduce the risk of electrical shock.
Use of electrical products in an environment for which they were not designed
can lead to fire or explosion. Fire regulations for the type of medical area being
used should be fully applied, observed, and enforced. Fire extinguishers should
be available for both electrical and nonelectrical fires.
Mechanical Safety
A list of precautions related to mechanical safety follows; observe these
precautions when using the system:
WARNINGS
• Be aware of the wheels on the system cart, especially when moving the
system. The system could cause injury to you or others if it rolls over feet
or into shins. Use caution when going up or down ramps.
• When attempting to overcome an obstacle, do not push the system from
either side with excessive force, which could cause the system to tip over.
• Position external hardcopy devices away from the system. Ensure that they
are secure. Do not stack them on the system.
CX50 User Manual
30
4535 616 38521
Safety
2

• When positioning the monitor, move it carefully to avoid pinching hands
or extremities against other objects, such as a bed rail.
• Never park the system on an incline.
• The brakes are intended as a convenience. To increase cart security, use
wheel chocks when the system is parked.
• If system operation is abnormal after you move or transport the system,
contact Philips Ultrasound Customer Service immediately. System
components are installed securely and can withstand considerable shock,
but excessive shock can cause a system failure.
• To avoid injury, Philips recommends against lifting the system cart.
CAUTIONS
• Before moving the system, ensure that the system is secured for transport.
On some systems, that may include ensuring that the monitor is latched,
to prevent monitor damage during transport.
• Ensure that the cables for all patient-applied parts are secure before moving
the system. Use the cable management system to ensure that transducer
cables are protected from damage.
• Do not roll the system over transducer cables or power cables.
• Do not use the system handle or transducer holders to move the cart.
• Never move the cart with the system on it, unless the system is properly
attached to the cart.
• To avoid the possibility of tipping the system cart when you move it over
a threshold, lift the cart slightly with the handle on the rear of the cart and
pull the cart over the threshold.
Equipment Protection
Follow these precautions to protect your system:
31
CX50 User Manual
4535 616 38521
2
Safety

CAUTIONS
• Excessive bending or twisting of cables on patient-applied parts may cause
failure or intermittent operation of the system. Do not roll the system over
cables, which may damage them.
• Improper cleaning or sterilization of a patient-applied part may cause
permanent damage. For cleaning and disinfection instructions, see the
«Transducer Care» section.
• Do not submerge the transducer connector in solution. The cables and
transducer bodies are liquid-tight, but the connectors are not.
• Do not use solvents, such as thinner or acetone, or abrasive cleaners on
the system, transducers, or any hardcopy device.
• For optimal performance, connect your ultrasound system to a circuit
dedicated solely for the system. Do not connect life-support devices to the
same circuit as the ultrasound system.
• If systems, transducers, and peripherals have been in an environment below
10°C (50°F), allow them to reach room temperature before connecting or
turning them on. Philips recommends allowing 24 hours for complete
normalization. Otherwise, condensation inside the devices could cause
damage. If the device was only briefly exposed to temperatures below 10°C
(50°F), then the time required for the device to return to room temperature
could be significantly less than 24 hours.
• To avoid damaging the flat-panel display in the monitor, do not store the
system where the ambient temperature exceeds 65°C (149°F).
Product Compatibility
Do not use your system in combination with other products or components,
unless Philips expressly recognizes those other products or components as
compatible. For information about such products and components, contact your
Philips representative.
Changes and additions to the system should be made only by Philips or by third
parties expressly authorized by Philips to do so. Such changes and additions must
CX50 User Manual
32
4535 616 38521
Safety
2

comply with all applicable laws and regulations that have the force of law within
the jurisdictions concerned, and best engineering practices.
WARNING
System changes and additions that are made without the appropriate training or
by using unapproved spare parts may void the Philips warranty. As with all
complex technical products, maintenance by unqualified persons or using
unapproved spare parts carries serious risks of system damage and personal
injury.
Symbols
The International Electrotechnical Commission (IEC) has established a set of
symbols for medical electronic equipment that classify a connection or warn of
potential hazards. Of those symbols, the following may be used on your Philips
product and its accessories and packaging.
Isolated patient connection (Type BF applied part).
Defibrillation-proof patient connection (Type BF
applied part).
Non-isolated patient connection (Type B applied
part).
Isolated patient connection for applied part intended
for intraoperative use, including direct cardiac
application and contact with major vessels (Type CF
applied part).
Defibrillation-proof patient connection (Type CF
applied part).
33
CX50 User Manual
4535 616 38521
2
Safety

Identifies ESD (electrostatic-discharge) sensitivity of
a connector that is not tested as specified in
IEC 60601-1-2. Do not touch exposed connector
pins. Touching exposed pins can cause electrostatic
discharge, which can damage the product.
Identifies an On/Off control.
On a two-position power switch, represents On ( )
and Off (
).
Identifies a safety note.
Indicates that the user should see the instructions
for use for safety information.
Identifies equipotential ground.
Identifies earth ground.
Identifies protective earth ground.
Nonionizing electromagnetic radiation. Indicates that
interference may occur in the vicinity of equipment
marked with this symbol.
The radio component contained in this device is
compliant to Council Directive 1999/5/EC (Radio
Equipment and Telecommunications Terminal
Equipment Directive).
CX50 User Manual
34
4535 616 38521
Safety
2

Indicates conformance with European Council
Directive 93/42/EEC.
Class 2 radio equipment identifier per Directive
1999/5/EC. European Union member states may apply
restrictions on putting this device into service or
placing it on the market. This device is intended to
be connected to the Publicly Available Interfaces for
use throughout the European Economic Area.
Indicates that the device is protected against the
effects of vertically falling water. This degree of
protection can apply to transducers or foot-operated
devices.
Indicates that the device is protected against the
effects of splashing liquids. This degree of protection
can apply to foot-operated devices.
Indicates that the device is protected against the
effects of immersion. This degree of protection can
apply to transducers and foot-operated devices.
Indicates that the device is protected against the
effects of immersion for up to 60 minutes. This
degree of protection can apply to foot-operated
devices.
35
CX50 User Manual
4535 616 38521
2
Safety

Indicates the need for separate collection for
electrical and electronic equipment in compliance
with the Waste Electrical and Electronic Equipment
(WEEE) Directive. When accompanied by
or ,
components of the device may contain lead or
mercury, respectively, which must be recycled or
disposed of in accordance with local, state, or federal
laws. The backlight lamps in an LCD system monitor
contain mercury.
Do not throw away. Dispose of in accordance with
local, state, or federal laws.
Do not reuse.
Use-by date.
Global Medical Device Nomenclature Code.
Indicates a possible crushing hazard to hands.
Warns that the system should not be used stacked
with other equipment. If the system is used stacked
with or adjacent to other equipment, verify normal
operation before use.
Indicates the temperature range (noncondensing) for
transport and storage. (Does not apply to media.)
CX50 User Manual
36
4535 616 38521
Safety
2

Indicates the atmospheric pressure range for
transport and storage.
Indicates the relative humidity range (noncondensing)
for transport and storage.
Indicates that a connector receives alternating
current.
Identifies fuse boxes or their locations. For continued
protection from fire and shock, replace fuses only
with fuses of the same type and rating.
Identifies the date of manufacture.
Identifies the legal manufacturer.
This side up: Points toward the side of the shipping
crate that should be kept facing up.
Indicates that the device should be kept dry.
Indicates that the device is fragile; handle with care.
Do not use if damaged.
Warns of system over-balance due to external force.
(Do not push on the monitor or the transducer
holders to move the system.)
37
CX50 User Manual
4535 616 38521
2
Safety

Keep away from sunlight.
Non-sterile.
Sterilized using ethylene oxide.
Catalog number.
Batch code.
Serial number.
Universal part number.
The following symbols may also be used on the system and its accessories and
packaging:
Connection for a pencil probe
Connection for a pencil probe
Connection for a transducer
CX50 User Manual
38
4535 616 38521
Safety
2

Connection for ECG leads
Connection for ECG leads
Print remote output
Input port for audio left/right, VHS/S-VHS,
microphone, CD, or DVD
Output port for audio left/right, VHS/S-VHS, video
patient monitor, black-and-white printer, or
interlaced RGB output port
Input port
VGA or parallel output port
DVI video output receptacle
USB input/output port
39
CX50 User Manual
4535 616 38521
2
Safety

FireWire (IEEE 1394) input/output port
Ethernet connection
RS-232 serial port
System microphone
Isolated auxiliary power provided for connection of
Philips-approved remote accessories.
Foot switch
Indicates the atmospheric pressure range for
transport and storage.
SVGA or DVI-I connection.
S-Video connection
CX50 User Manual
40
4535 616 38521
Safety
2

B/W Composite video output connection
Color composite video output connection
Video print trigger connection
Russian approval (GOST)
Identifies the port for the PercuNav tool connection
unit.
Identifies the port for the PercuNav field generator.
Chinese Environmentally Friendly Use Period symbol.
UL (Underwriters Laboratories) classification symbol.
CSA (CSA International) classification symbol.
Indicates a possible pinch hazard when positioning
the monitor.
41
CX50 User Manual
4535 616 38521
2
Safety

The following symbols may be used inside the system:
Dangerous voltages: Appears adjacent to high-voltage
terminals, indicating the presence of voltages greater
than 1,000 Vac (600 Vac in the United States).
Identifies equipotential ground.
Biological Safety
This section contains information about biological safety and a discussion of the
prudent use of the system.
A list of precautions related to biological safety follows; observe these precautions
when using the system. For more information refer to Medical Ultrasound Safety
on your user information CD.
WARNINGS
• Do not use the system if an error message on the video display indicates
that a hazardous condition exists. Note the error code, turn off power to
the system, and call your customer service representative.
• Do not use a system that exhibits erratic or inconsistent image updating.
Discontinuities in the scanning sequence indicate a hardware failure that
must be corrected before use.
• Perform ultrasound procedures prudently. Use the ALARA (as low as
reasonably achievable) principle.
• Use only acoustic standoffs that have been approved by Philips Ultrasound.
For information on ordering approved accessories, see
«Supplies and
Accessories» on page 21
.
• Verify the alignment of the biopsy guide before use. See the
«Biopsy Guides»
section.
• Verify the condition of the biopsy needle before use. Do not use a bent
biopsy needle.
CX50 User Manual
42
4535 616 38521
Safety
2

• Transducer covers may contain natural rubber latex. Those covers may
cause allergic reactions in some individuals. See
«FDA Medical Alert on
Latex» on page 44
.
• The M2203A bite guard strap contains natural rubber latex, which may
cause allergic reactions. See
«FDA Medical Alert on Latex» on page 44.
• In contrast studies using a high-MI acoustic field, capillary rupture, due to
microbubble expansion within a capillary in an acoustic field, can cause
extravasation. References: (1) Skyba, D.M., Price, R.J., Linka, A.Z., Skalak,
T.C., Kaul, S. «Direct in vivo visualization of intravascular destruction of
microbubbles by ultrasound and its local effects on tissue.» Circulation, 1998;
98:290-293. (2) van Der Wouw, P.A., Brauns, A.C., Bailey, S.E., Powers, J.E.,
Wilde, A.A. «Premature ventricular contractions during triggered imaging
with ultrasound contrast.» Journal of the American Society of Echocardiography,
2000;13(4):288-94.
• Preventricular contractions can be caused by the oscillations of microbubbles
when a high-MI acoustic field is triggered in the heart at the end of systole.
In a very sick patient with certain risk factors, theoretically, this could lead
to ventricular fibrillation. Reference: van Der Wouw, P.A., Brauns, A.C.,
Bailey, S.E., Powers, J.E., Wilde, A.A. «Premature ventricular contractions
during triggered imaging with ultrasound contrast.» Journal of the American
Society of Echocardiography, 2000;13(4):288-94.
• If a sterile transducer cover becomes compromised during an intraoperative
application involving a patient with transmissible spongiform encephalopathy,
such as Creutzfeldt-Jakob disease, follow the guidelines of the U.S. Centers
for Disease Control and this document from the World Health Organization:
WHO/CDS/ APH/2000/3, WHO Infection Control Guidelines for
Transmissible Spongiform Encephalopathies. The transducers for your
system cannot be decontaminated using a heat process.
• If the system becomes contaminated internally with bodily fluids carrying
pathogens, you must immediately notify your Philips service representative.
Components inside the system cannot be disinfected. In that case, the
system must be disposed of as biohazardous material in accordance with
local or federal laws.
43
CX50 User Manual
4535 616 38521
2
Safety

• The backlight lamps in the system displays contain mercury and must be
recycled or disposed of according to local, state, or federal laws.
• Select the correct application when starting an exam, and remain in that
application throughout the exam. Some applications are for parts of the
body that require lower limits for acoustic output. One example is an
ophthalmic application activated by selecting an orbital transcranial Doppler
preset; when performing an ophthalmic exam, use only an ophthalmic preset.
• When used off the cart, the AC adapter and the system should not be
placed on the floor or on a patient’s bed. You can place it on a table or
chair.
FDA Medical Alert on Latex
March 29, 1991, Allergic Reactions to Latex-Containing Medical Devices
Because of reports of severe allergic reactions to medical devices containing
latex (natural rubber), the FDA is advising health care professionals to identify
their latex sensitive patients and be prepared to treat allergic reactions promptly.
Patient reactions to latex have ranged from contact urticaria to systemic
anaphylaxis. Latex is a component of many medical devices, including surgical
and examination gloves, catheters, intubation tubes, anesthesia masks, and dental
dams.
Reports to the FDA of allergic reactions to latex-containing medical devices have
increased lately. One brand of latex cuffed enema tips was recently recalled after
several patients died as a result of anaphylactoid reactions during barium enema
procedures. More reports of latex sensitivity have also been found in the medical
literature. Repeated exposure to latex both in medical devices and in other
consumer products may be part of the reason that the prevalence of latex
sensitivity appears to be increasing. For example, it has been reported that 6%
to 7% of surgical personnel and 18% to 40% of spina bifida patients are latex
sensitive.
Proteins in the latex itself appear to be the primary source of the allergic
reactions. Although it is not now known how much protein is likely to cause
CX50 User Manual
44
4535 616 38521
Safety
2

severe reactions, the FDA is working with manufacturers of latex-containing
medical devices to make protein levels in their products as low as possible.
FDA’s recommendations to health professionals in regard to this problem are
as follows:
• When taking general histories of patients, include questions about latex
sensitivity. For surgical and radiology patients, spina bifida patients and health
care workers, this recommendation is especially important. Questions about
itching, rash or wheezing after wearing latex gloves or inflating a toy balloon
may be useful. Patients with positive histories should have their charts
flagged.
• If latex sensitivity is suspected, consider using devices made with alternative
materials, such as plastic. For example, a health professional could wear a
non-latex glove over the latex glove if the patient is sensitive. If both the
health professional and the patient are sensitive, a latex middle glove could
be used. (Latex gloves labeled “Hypoallergenic” may not always prevent
adverse reactions.)
• Whenever latex-containing medical devices are used, especially when the
latex comes in contact with mucous membranes, be alert to the possibility
of an allergic reaction.
• If an allergic reaction does occur and latex is suspected, advise the patient
of a possible latex sensitivity and consider an immunologic evaluation.
• Advise the patient to tell health professionals and emergency personnel
about any known latex sensitivity before undergoing medical procedures.
Consider advising patients with severe latex sensitivity to wear a medical
identification bracelet.
The FDA is asking health professionals to report incidents of adverse reactions
to latex or other materials used in medical devices. (See the October 1990 FDA
Drug Bulletin.) To report an incident, contact the FDA Problem Reporting
Program, MedWatch, at 1-800-332-1088, or on the Internet:
www.fda.gov/Safety/MedWatch/
For a single copy of a reference list on latex sensitivity, write to: LATEX, FDA,
HFZ-220, Rockville, MD 20857.
45
CX50 User Manual
4535 616 38521
2
Safety

NOTE
The ultrasound system and transducers described in this document do not
contain natural rubber latex that contacts humans. Natural rubber latex is not
used on any Philips ultrasound transducer. It also is not used on Philips ECG
cables for the products described in this document.
ALARA Education Program
The guiding principle for the use of diagnostic ultrasound is defined by the «as
low as reasonably achievable» (ALARA) principle. The decision as to what is
reasonable has been left to the judgment and insight of qualified personnel. No
set of rules can be formulated that would be sufficiently complete to dictate the
correct response to every circumstance. By keeping ultrasound exposure as low
as possible, while obtaining diagnostic images, users can minimize ultrasonic
bioeffects.
Since the threshold for diagnostic ultrasound bioeffects is undetermined, it is
the sonographer’s responsibility to control total energy transmitted into the
patient. The sonographer must reconcile exposure time with diagnostic image
quality. To ensure diagnostic image quality and limit exposure time, an ultrasound
system provides controls that can be manipulated during the exam to optimize
the results of the exam.
The ability of the user to abide by the ALARA principle is important. Advances
in diagnostic ultrasound, not only in the technology but in the applications of
that technology, have resulted in the need for more and better information to
guide the user. The output display indices are designed to provide that important
information.
There are a number of variables which affect the way in which the output display
indices can be used to implement the ALARA principle. These variables include
index values, body size, location of the bone relative to the focal point, attenuation
in the body, and ultrasound exposure time. Exposure time is an especially useful
variable, because it is controlled by the user. The ability to limit the index values
over time supports the ALARA principle.
CX50 User Manual
46
4535 616 38521
Safety
2

Applying ALARA
The system imaging mode used depends upon the information needed. 2D and
M-mode imaging provide anatomical information, while Doppler, Color Power
Angio (CPA), and Color imaging provide information about blood flow. A scanned
mode, like 2D or Color, disperses or scatters the ultrasonic energy over an area,
while an unscanned mode, like M-mode or Doppler, concentrates ultrasonic
energy. Understanding the nature of the imaging mode being used allows the
sonographer to apply the ALARA principle with informed judgment. Additionally,
the transducer frequency, system setup values, scanning techniques, and operator
experience allow the sonographer to meet the definition of the ALARA principle.
The decision as to the amount of acoustic output is, in the final analysis, up to
the system operator. This decision must be based on the following factors: type
of patient, type of exam, patient history, ease or difficulty of obtaining
diagnostically useful information, and the potential localized heating of the patient
due to transducer surface temperatures. Prudent use of the system occurs when
patient exposure is limited to the lowest index reading for the shortest amount
of time necessary to achieve acceptable diagnostic results.
Although a high index reading does not mean that a bioeffect is actually occurring,
a high index reading should be taken seriously. Every effort should be made to
reduce the possible effects of a high index reading. Limiting exposure time is an
effective way to accomplish this goal.
There are several system controls that the operator can use to adjust the image
quality and limit the acoustic intensity. These controls are related to the
techniques that an operator might use to implement ALARA. These controls
can be divided into three categories: direct, indirect, and receiver controls.
Acoustic Output Limits
This ultrasound system maintains acoustic output below the appropriate limits
for each application, as listed here. The significant difference in magnitude
emphasizes the need to select the correct application and remain in that
application, so the correct application limits are in use for the appropriate
application.
47
CX50 User Manual
4535 616 38521
2
Safety

Limits for Non-Ophthalmic Applications
• I
spta.3
≤ 720 mW/cm
2
• MI ≤ 1.9
• TI ≤ 6.0
Limits for Ophthalmic Applications
• I
spta.3
≤ 50 mW/cm
2
• MI ≤ 0.23
• TI ≤ 1.0
Direct Controls
Application selection and the output-power control directly affect acoustic
intensity. There are different ranges of allowable intensity or output based on
your selection. Selecting the correct range of acoustic intensity for the application
is one of the first things that occurs in any exam. For example, peripheral vascular
intensity levels are not recommended for fetal exams. Some systems automatically
select the proper range for a particular application, while others require manual
selection. Ultimately, the user has the responsibility for proper clinical use. The
ultrasound system provides both automatic (default) settings and manual
(user-selectable) settings.
Output power has direct impact on acoustic intensity. Once the application has
been established, the power control can be used to increase or decrease the
intensity output. The power control allows you to select intensity levels less
than the established maximum. Prudent use dictates that you select the lowest
output intensity that is consistent with good image quality.
Indirect Controls
The indirect controls are those that have an indirect effect on acoustic intensity.
These controls affect imaging mode, pulse repetition frequency, focus depth,
pulse length, and transducer selection.
The choice of imaging mode determines the nature of the ultrasound beam. 2D
is a scanning mode; Doppler is a stationary or unscanned mode. A stationary
ultrasound beam concentrates energy in a single location. A moving or scanned
CX50 User Manual
48
4535 616 38521
Safety
2

ultrasound beam disperses the energy over an area and the beam is concentrated
on the same area for a fraction of the time as that of an unscanned mode.
Pulse repetition frequency or rate refers to the number of ultrasound bursts of
energy over a specific period of time. The higher the pulse repetition frequency,
the more pulses of energy in a period of time. Several controls affect pulse
repetition frequency: focal depth, display depth, sample volume depth, flow
optimization, scale, number of focal zones, and sector-width controls.
Focus of the ultrasound beam affects the image resolution. To maintain or
increase resolution at a different focus requires a variation in output over the
focal zone. This variation of output is a function of system optimization. Different
exams require different focal depths. Setting the focus at the proper depth
improves the resolution of the structure of interest.
Pulse length is the time during which the ultrasonic burst is turned on. The longer
the pulse, the greater the time-average intensity value. The greater the
time-average intensity, the greater the likelihood of temperature increase and
cavitation. Pulse length, burst length, or pulse duration is the output pulse
duration in PW Doppler. Increasing the Doppler sample-volume size increases
the pulse length.
Transducer selection indirectly affects intensity. Tissue attenuation changes with
frequency. The higher the transducer operating frequency, the greater the
attenuation of the ultrasonic energy. A higher transducer operating frequency
requires more output intensity to scan at a deeper depth. To scan deeper at the
same output intensity, a lower transducer frequency is required. Using more
gain and output beyond a point, without corresponding increases in image quality,
can mean that a lower frequency transducer is needed.
Receiver Controls
Receiver controls are used by the operator to improve image quality. These
controls have no effect on output. Receiver controls only affect how the
ultrasound echo is received. These controls include gain, TGC, dynamic range,
and image processing. The important thing to remember, relative to output, is
that receiver controls should be optimized before output is increased. For
example, before increasing output, optimize gain to improve image quality.
49
CX50 User Manual
4535 616 38521
2
Safety

An Example of Applying the ALARA Principle
An ultrasound scan of a patient’s liver begins with selecting the appropriate
transducer frequency. After selecting the transducer and the application, which
are based on patient anatomy, adjustments to output power should be made to
ensure that the lowest possible setting is used to acquire an image. After the
image is acquired, adjusting the focus of the transducer, and then increasing the
receiver gain to produce a uniform representation of the tissue follows. If an
adequate image can be obtained with the increase in gain, then a decrease in
output should be made. Only after making these adjustments should you increase
output to the next level.
Having acquired the 2D display of the liver, Color can be used to localize blood
flow. As with the 2D image display, gain and image processing controls must be
optimized before increasing output.
Having localized the blood flow, use the Doppler controls to position the sample
volume over the vessel. Before increasing output, adjust velocity range or scale
and Doppler gain to obtain an optimal Doppler trace. Only if maximum Doppler
gain does not create an acceptable image do you increase output.
In summary: Select the correct transducer frequency and application for the job;
start with a low output level; and optimize the image by using focus, receiver
gain, and other imaging controls. If the image is not diagnostically useful at this
point, then increase output.
Additional Considerations
Ensure that scanning time is kept to a minimum, and ensure that only medically
required scanning is performed. Never compromise quality by rushing through
an exam. A poor exam may require a follow-up, which ultimately increases
exposure time. Diagnostic ultrasound is an important tool in medicine, and like
any tool, it should be used efficiently and effectively.
Output Display
The system output display comprises two basic indices: a mechanical index and
a thermal index. The thermal index further consists of the following indices: soft
CX50 User Manual
50
4535 616 38521
Safety
2
 Loading…
Loading…
Портативный УЗ-комплекс Philips CX50 — современная и многофункциональная ультразвуковая платформа, необходимая для реализации диагностических исследований в стационарных условиях или во время выезда непосредственно к местоположению пациента.
Главные преимущества ультразвукового аппарата Philips CX50
— яркий экран с поддержкой высокого разрешения и диагональю в 15,4 дюймов,
— удобная клавиатура с клавишами быстрого управления,
— 10 разновидностей компенсаторного управления: 2 по TGC и 2 по LGC,
— 2 активных слота для USB,
— объем жесткого диска в размере 80 Гб,
— использует современные инструменты: Live 3D-TEE, x-Matrix, x-Stream, x-Plane, Strain-Rate, GI-3DQ, i-FOCUS,i-SCAN, i-Optimize, Sono-CT, Q-Lab, High-Q, Live i-Slice, TSI, DICOM и другие,
— опциональное расширение с помощью инновационных разработок Purewave, Sono-Ct, Xres и т.д.,
— высокие показатели достоверности 3D-диагностики благодаря возможностям 3D-Freehand,
— виды датчиков-измерителей: линейные, внутриполостные, конвексные, матричные, интраоперационные и т.д.
УЗИ-аппарат Philips CX50 подходит для работы в различных медицинских организациях в области: педиатрии, урологии, эхокардиографии (для взрослых и детей), абдоминальной хирургии.
Знакомство с новейшей компактной УЗ системой Philips
Теперь вы можете быть уверены 8 результатах своих исследований, даже самых сложных в техническом отношении. Компания Philips, лидирующая в области разработок самого современного ультразвукового оборудования, объединила передовые инновационные технологии в новой системе СХ50, призванной удовлетворить потребности в высокоэффективных компактных эхосистемах.
„Проводя исследования у постели больного или на выезде, я не всегда могу рассчитывать на компактную ультразвуковую систему, когда имею дело с крупными или сложными для сканирования пациентами. Иногда мне приходится заново проводить исследования с помощью высококлассной эхосистемы или прибегать к другому методу. Мне необходима возможность быстрой и точной постановки диагноза у постели пациента.»
У постели больного
Компактные размеры прибора СХ50 позволяют проводить высококлассные исследования непосредственно у постели больного.
Операционная
Мобильность системы СХ50 означает возможность высококачественной чреспищеводной эхокардиографии в операционной.
Отделение кардиореанимации
Благодаря отличному качеству изображения система СХ50 идеально подходит для обследования пациентов, находящихся в критическом состоянии.
Высокая эффективность компактной эхосистемы
Отличные технические характеристики, реализованные в системе СХ50, объединены с высокоэффективными, апробированными в клинических условиях технологиями. Изображения исключительной четкости позволяют получать данные, необходимые для постановки точных диагнозов.
Высококлассная компактная эхосистема всегда под рукой
Система СХ50 подстраивается под ваш рабочий процесс, где бы вы ни находились: в отделении кардиореанимации, у постели больного в стационаре или на выезде. Простые в использовании инструменты, разработанные с учетом потребностей врачей, позволяют обследовать пациентов вне зависимости от их местонахождения.
Революционные решения для оптимизации рабочего процесса
Выездные исследования стали проще благодаря простым элементам управления. Система CX50 была создана для того, чтобы сделать выездные исследования проще и эффективнее.
Технология iSCAN обеспечивает автоматическую выборку данных для нового уровня оптимизации 2D и доплеровского режимов. Работая с СХ50. вы можете полностью положиться на качество визуализации и доплеровских исследований для всех своих пациентов. Элемента управления функциями прибора логично размещена на панели управления СХ50, обеспечивая быстроту выбора и оптимизации в ходе каждого исследования.
Окончательная обработка данных исследований с помощью активных исходных данных
Система СХ50 сохраняет активные исходные акустические данные и позволяет обрабатывать практически все параметры сканирования на отдельных изображениях, фрагментах или сохраненных 2D и доплеровских данных. Изображения можно корректировать как в ходе исследования, так и после него, что позволяет снизить продолжительность исследований и потребность в их повторе.
Увеличение диагностической информации с помощью программы количественного анализа QLAB
Система СХ50 предоставляет количественную оценку анатомии и функции сердца при помощи клинически апробированного программного обеспечения QLAB. Поддерживаются следующие
модули: количественный анализ движения ткани (TMQ) с технологией отслеживания дифракционных пятен, количественный анализ деформации миокарда (SQ) и анализ области исследования (ROI).
Компактный ультразвуковой прибор, рассчитанный на любые условия работы
Система CX50 оснащена 15-дюймовым монитором с высоким разрешением для оптимального просмотра изображений практически в любых, в том числе самых сложных, условиях вне стационара, а быстрый запуск системы позволяет немедленно приступить к исследованию. Возможность как проводного, так и беспроводного подключения к системе PACS в формате DICOM повышает универсальность прибора. С помощью средства просмотра DICOM можно экспортировать данные на DVD и USB носители.
– Работа в полевых условиях
Систему CX50 легко доставить на место проведения скрининга, в район стихийного бедствия, в сельскую местность или в любое другое место, где необходимо провести качественное ультразвуковое исследование и получить информативные и точные результаты.
– Удаленная работа
Система CX50 идеально подходит для проведения исследований в удалённых кабинетах. Перевозя её в специальной сумке, можно без труда обеспечить потребности населения определенной области в качественных ультразвуковых исследованиях.
– У постели больного
Благодаря своим компактным размерам система CX50 отлично подходит для проведения исследований в ограниченном пространстве. У вас не возникнет трудностей при проведении УЗИ сердца и сосудов непосредственно у постели тяжелобольного пациента.
– Отделения реанимации
Благодаря высокому качеству изображений система CX50 идеально подходит для проведения УЗИ тяжелобольных. Ее малый вес, небольшие размеры и тележка на колесах позволяют легко маневрировать в ограниченном пространстве отделения реанимации.
– ОРИТ новорожденных
Возможности системы CX50 позволяют обеспечить быструю и качественную визуализацию самых маленьких пациентов в критическом состоянии. Систему CX50 можно без труда перемещать в пределах ОРИТ новорожденных, при этом высокое качество визуализации позволит выделить тончайшие структуры, необходимые для надежной диагностики.
– Ангиографический кабинет
Благодаря компактности и превосходному качеству изображений системы CX50 xMATRIX ее интеграция с рентгенографическим аппаратом Philips Allura является уникальным решением для эхокардиографической навигации во время основных и комбинированных интервенционных процедур.
– Операционная
Система CX50 xMATRIX оснащена технологией Live 3D TEE, которая поддерживается чреспищеводным датчиком X7-2t. Компактный размер системы упрощает проведение чреспищеводной визуализации в операционной для предоперационного анализа и постоперационной оценки результатов различных процедур, включая операции на клапанах. Чреспищеводный датчик X7-2t для системы CX50 также можно использовать на системе iE33 xMATRIX, что существенно повышает его клиническую и эксплуатационную эффективность.
Портативная система CX50 отличается превосходными рабочими характеристиками, которые позволяют проводить ультразвуковые исследования проще, чем когда-либо ранее. Компания Philips перенесла на мобильную платформу высококлассные и клинически проверенные технологии. Система способна адаптироваться к вашим способам работы, а ее функции разработаны с учетом различных рабочих условий: в отделении реанимации, у постели больного или вне лечебного учреждения. В результате в ваших руках окажется переносная ультразвуковая система, отличающаяся простотой использования, превосходным качеством изображений и специализированными средствами анализа.
– Технология PureWave там, где это необходимо
Технология PureWave, которая раньше поддерживалась только в системе Philips iE33, была интегрирована в новую систему CX50 xMATRIX. Эта система предлагает врачам превосходное разрешение и возможность исследования пациентов различных категорий с помощью одного и того же датчика. Революционная технология создания пьезоэлектрических датчиков PureWave была отмечена международной наградой и стала принципиально новой вехой в обеспечении качества изображений на компактных системах. С помощью этой технологии уже проведены миллионы исследований. И это только часть из того, чем вы можете воспользоваться при проведении УЗИ.
– Широкополосная цифровая технология формирования луча на компактной ультразвуковой системе
Система CX50 сочетает в себе широкополосную цифровую технологию формирования луча с возможностью генерации широкополосных сигналов с помощью датчиков PureWave. Теперь вы можете даже с помощью компактной системы получить, сохранить и вывести на экран полную информацию об исследуемой ткани. При этом высочайший уровень качества изображений позволит вам без труда рассмотреть даже мельчайшие анатомические детали.
– Технологии SonoCT и XRES – новый уровень визуализации в компактной ультразвуковой системе
Клинически проверенная современная технология Philips SonoCT позволяет получать до девяти изображений под разными углами обзора и объединять их в режиме реального времени в единое изображение высокого разрешения. Технология SonoCT обеспечивает превосходную дифференциацию тканей и исключает практически все артефакты. Улучшенная адаптивная технология обработки изображений XRES подавляет зернистость, размытости и помехи в изображениях, благодаря чему они практически не содержат шумов и отличаются потрясающей четкостью и резкостью контуров. Совместное применение технологий SonoCT и XRES позволяет увидеть даже мельчайшие детали, необходимые для диагностики, и обеспечить высокую клиническую точность результатов.
– Больше информации для планирования
Технология Live 3D TEE предоставляет хирургам и анестезиологам большой объем информации, облегчающий планирование хирургических операций. Она позволяет получить необходимые оценки по трехмерным изображениям работающего сердца. В дополнение к этому, из набора трехмерных данных можно получить множество двумерных изображений срезов, что позволяет уточнить информацию о различных дефектах (например, клапанов и их створок). Передние проекции и изображение левого желудочка (недоступные при трансторакальном исследовании) открывают существенно больше возможностей для планирования. Эти изображения уже невозможно получить после начала хирургической операции.
– Количественный анализ митрального клапана с помощью новых объективных данных
Функция количественной оценки митрального клапана (MVQ) позволяет проводить измерения митрального клапана и соседних структур по изображениям мультипланарной реконструкции (MPR), проводимой на основе трехмерных данных, полученных в режиме Live 3D TEE. Это обеспечивает поддержку для принятия клинических решений при планировании хирургической операции. При этом функция MVQ предоставляет протоколы для определения трехмерных анатомических маркеров на MPR-изображениях. Кроме того, функция MVQ позволяет построить трехмерную модель аорты, отверстия и створок митрального клапана, которая помогает понять пространственную взаимосвязь между митральным клапаном, папиллярными мышцами и аортальным клапаном.
Дополнительная информация
